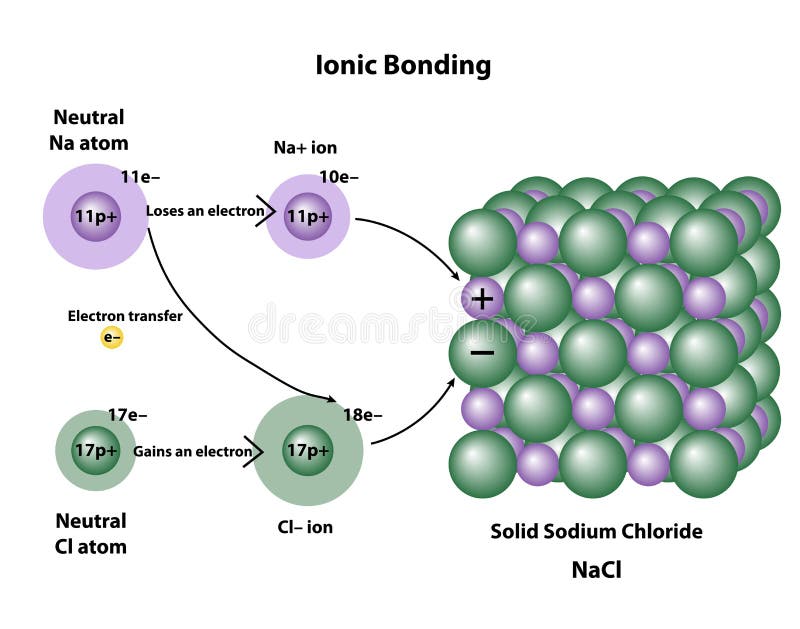
Is Table Salt A Covalent Bond . The ionic compound sodium chloride, also known as salt, has. examples of compounds with ionic bonds include salt, such as table salt (nacl). the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. to tell if nacl (table salt) is ionic or covalent (also called molecular) we. the simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and. It forms an ionic bond. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. nacl is not a covalent bond. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. ionic bonding in sodium chloride. In salt, the sodium atom donates its electron, so it yields the. We’ll talk about what is an ionic.
from www.dreamstime.com
We’ll talk about what is an ionic. The ionic compound sodium chloride, also known as salt, has. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. ionic bonding in sodium chloride. the simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and. examples of compounds with ionic bonds include salt, such as table salt (nacl). It forms an ionic bond. In salt, the sodium atom donates its electron, so it yields the. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting.
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector
Is Table Salt A Covalent Bond ionic bonding in sodium chloride. We’ll talk about what is an ionic. In salt, the sodium atom donates its electron, so it yields the. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. It forms an ionic bond. ionic bonding in sodium chloride. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. the simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and. examples of compounds with ionic bonds include salt, such as table salt (nacl). nacl is not a covalent bond. The ionic compound sodium chloride, also known as salt, has. to tell if nacl (table salt) is ionic or covalent (also called molecular) we.
From www.youtube.com
Is Table Salt (NaCl ) Ionic or Covalent/Molecular? YouTube Is Table Salt A Covalent Bond in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. It forms an ionic bond. In salt, the sodium atom donates its electron, so it yields the. the simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and. We’ll talk about. Is Table Salt A Covalent Bond.
From www.pikpng.com
Download Png Royalty Free Drawing Atoms Sodium Covalent Bonding Of Is Table Salt A Covalent Bond In salt, the sodium atom donates its electron, so it yields the. ionic bonding in sodium chloride. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. . Is Table Salt A Covalent Bond.
From www.chemistrystudent.com
Covalent and Ionic Character (ALevel) ChemistryStudent Is Table Salt A Covalent Bond It forms an ionic bond. the simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and. nacl is not a covalent bond. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. The ionic compound sodium chloride, also known as. Is Table Salt A Covalent Bond.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Is Table Salt A Covalent Bond the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. examples of compounds with ionic bonds include salt, such as table salt (nacl). to tell if nacl (table salt) is ionic or covalent (also called molecular) we. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs. Is Table Salt A Covalent Bond.
From kotop.shinbroadband.com
Exploring Nacl The Ionic Compound Wonder Is Table Salt A Covalent Bond In salt, the sodium atom donates its electron, so it yields the. The ionic compound sodium chloride, also known as salt, has. nacl is not a covalent bond. examples of compounds with ionic bonds include salt, such as table salt (nacl). the simplist guide to the covalent or ionic character of a bond is to consider the. Is Table Salt A Covalent Bond.
From owlcation.com
Primary and Secondary Bonds Owlcation Is Table Salt A Covalent Bond It forms an ionic bond. The ionic compound sodium chloride, also known as salt, has. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. examples of compounds with ionic bonds include salt, such as table. Is Table Salt A Covalent Bond.
From exoilfotb.blob.core.windows.net
What Is Table Salt In Chemistry at Christina Stotts blog Is Table Salt A Covalent Bond the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. examples of compounds with ionic bonds include salt, such as table salt (nacl). whereas ionic compounds are usually formed when a metal and a nonmetal. Is Table Salt A Covalent Bond.
From exomdamui.blob.core.windows.net
Table Salt Atomic Formula at Pete Alvarez blog Is Table Salt A Covalent Bond whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. We’ll talk about what is an ionic. examples of compounds with ionic bonds include salt, such as table salt (nacl). the simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved. Is Table Salt A Covalent Bond.
From courses.lumenlearning.com
Why Life Depends on Water Biology for NonMajors I Is Table Salt A Covalent Bond nacl is not a covalent bond. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. ionic bonding in sodium chloride. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. the simplist guide to the covalent or ionic character of a bond is. Is Table Salt A Covalent Bond.
From cabinet.matttroy.net
Is Table Salt An Ionic Or Molecular Compound Matttroy Is Table Salt A Covalent Bond ionic bonding in sodium chloride. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. In salt, the sodium atom donates its electron, so it yields the. examples of compounds with ionic bonds include salt, such as table salt (nacl). in this article, we discuss ionic bonding and covalent bonding, and compare. Is Table Salt A Covalent Bond.
From chemistrylhs.weebly.com
GCSE ChemistryLHS Is Table Salt A Covalent Bond The ionic compound sodium chloride, also known as salt, has. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. We’ll talk about what is an ionic. in. Is Table Salt A Covalent Bond.
From www.thoughtco.com
Examples of Covalent Bonds and Compounds Is Table Salt A Covalent Bond We’ll talk about what is an ionic. It forms an ionic bond. ionic bonding in sodium chloride. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. The ionic compound sodium chloride, also known as salt, has. In salt, the sodium atom donates its electron, so it yields the. the. Is Table Salt A Covalent Bond.
From cabinet.matttroy.net
Table Salt Nacl Is A Molecular Compound True Or False Matttroy Is Table Salt A Covalent Bond In salt, the sodium atom donates its electron, so it yields the. ionic bonding in sodium chloride. It forms an ionic bond. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the. Is Table Salt A Covalent Bond.
From sciencenotes.org
Covalent Compounds Examples and Properties Is Table Salt A Covalent Bond the simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. ionic bonding in sodium chloride. The ionic compound sodium chloride, also known as salt, has. It forms an ionic. Is Table Salt A Covalent Bond.
From www.politicalfunda.com
Covalent Bond Covalent Bond Definition, Types, Properties & Facts Is Table Salt A Covalent Bond in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. The ionic compound sodium chloride, also known as salt, has. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. ionic bonding in sodium chloride. nacl is not a covalent bond. We’ll talk about what. Is Table Salt A Covalent Bond.
From www.slideshare.net
Chemistry of Life Is Table Salt A Covalent Bond in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. ionic bonding in sodium chloride. to tell if nacl (table salt) is ionic or covalent (also called molecular) we. The ionic compound. Is Table Salt A Covalent Bond.
From thescienceandmathszone.com
Covalent Bonding The Science and Maths Zone Is Table Salt A Covalent Bond to tell if nacl (table salt) is ionic or covalent (also called molecular) we. nacl is not a covalent bond. In salt, the sodium atom donates its electron, so it yields the. examples of compounds with ionic bonds include salt, such as table salt (nacl). whereas ionic compounds are usually formed when a metal and a. Is Table Salt A Covalent Bond.
From informacionpublica.svet.gob.gt
Examples Of Covalent Bonds And Compounds Is Table Salt A Covalent Bond ionic bonding in sodium chloride. nacl is not a covalent bond. It forms an ionic bond. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. examples of compounds with ionic. Is Table Salt A Covalent Bond.
From www.thoughtco.com
Chemical Composition of Table Salt Is Table Salt A Covalent Bond whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. We’ll talk about what is an ionic. to tell if nacl (table salt) is ionic or covalent (also called molecular) we. ionic bonding in sodium chloride. The ionic compound sodium chloride, also known as salt, has. the sodium and. Is Table Salt A Covalent Bond.
From infinitylearn.com
Covalent Bond Important Topic of Chemistry Is Table Salt A Covalent Bond We’ll talk about what is an ionic. The ionic compound sodium chloride, also known as salt, has. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. the simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and. ionic bonding. Is Table Salt A Covalent Bond.
From ar.inspiredpencil.com
Covalent Bond Examples Is Table Salt A Covalent Bond whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. It forms an ionic bond. to tell if nacl (table salt) is ionic or covalent (also called molecular) we. ionic bonding in sodium chloride. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs. Is Table Salt A Covalent Bond.
From readingandwritingprojectcom.web.fc2.com
the bond in table salt (nacl) is Is Table Salt A Covalent Bond whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. It forms an ionic bond. examples of compounds with ionic bonds include salt, such as table salt (nacl). The ionic compound sodium chloride, also known as salt, has. nacl is not a covalent bond. We’ll talk about what is an. Is Table Salt A Covalent Bond.
From www.politicalfunda.com
Covalent Bond Covalent Bond Definition, Types, Properties & Facts Is Table Salt A Covalent Bond whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. It forms an ionic bond. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. in this article, we discuss ionic bonding and covalent bonding, and compare ionic. Is Table Salt A Covalent Bond.
From fity.club
Examples Of Covalent Compounds Is Table Salt A Covalent Bond the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. In salt, the sodium atom donates its electron, so it yields the. It forms an ionic bond. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. examples of compounds with ionic bonds include salt, such. Is Table Salt A Covalent Bond.
From brainly.com
This image shows the ionic bond between sodium and chloride in NaCl Is Table Salt A Covalent Bond whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. We’ll talk about what is an ionic. nacl is not a covalent bond. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. to tell if nacl (table salt) is ionic or covalent (also called. Is Table Salt A Covalent Bond.
From www.slideserve.com
PPT INTRO TO CHEMISTRY PowerPoint Presentation, free download ID Is Table Salt A Covalent Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. We’ll talk about what is an ionic. to tell if nacl (table salt) is ionic or covalent (also called molecular) we. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent. Is Table Salt A Covalent Bond.
From www.istockphoto.com
Ionic Vs Covalent Bonds Stock Illustration Download Image Now Is Table Salt A Covalent Bond whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. The ionic compound sodium chloride, also known as salt, has. It forms an ionic bond. the simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and. the sodium and chlorine. Is Table Salt A Covalent Bond.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector Is Table Salt A Covalent Bond In salt, the sodium atom donates its electron, so it yields the. ionic bonding in sodium chloride. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. . Is Table Salt A Covalent Bond.
From www.adda247.com
Covalent Bond Definition, Examples for Class 10 Is Table Salt A Covalent Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. examples of compounds with ionic bonds include salt, such as table salt (nacl). to tell if nacl (table salt) is ionic or covalent (also called molecular) we. the simplist guide to the covalent or. Is Table Salt A Covalent Bond.
From studymind.co.uk
Covalent Bond Diagrams (GCSE Chemistry) Study Mind Is Table Salt A Covalent Bond the simplist guide to the covalent or ionic character of a bond is to consider the types of atoms involved and. to tell if nacl (table salt) is ionic or covalent (also called molecular) we. examples of compounds with ionic bonds include salt, such as table salt (nacl). We’ll talk about what is an ionic. nacl. Is Table Salt A Covalent Bond.
From mungfali.com
Covalent Bond Periodic Table Is Table Salt A Covalent Bond nacl is not a covalent bond. in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. The ionic compound sodium chloride, also known as salt, has. We’ll talk about what is an ionic. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually.. Is Table Salt A Covalent Bond.
From exoxnecug.blob.core.windows.net
Table Salt Sodium Chloride Elements at Francisco White blog Is Table Salt A Covalent Bond examples of compounds with ionic bonds include salt, such as table salt (nacl). to tell if nacl (table salt) is ionic or covalent (also called molecular) we. It forms an ionic bond. The ionic compound sodium chloride, also known as salt, has. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. . Is Table Salt A Covalent Bond.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Is Table Salt A Covalent Bond We’ll talk about what is an ionic. to tell if nacl (table salt) is ionic or covalent (also called molecular) we. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting.. Is Table Salt A Covalent Bond.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Is Table Salt A Covalent Bond nacl is not a covalent bond. to tell if nacl (table salt) is ionic or covalent (also called molecular) we. whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually. the sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to. It forms an ionic bond.. Is Table Salt A Covalent Bond.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Is Table Salt A Covalent Bond in this article, we discuss ionic bonding and covalent bonding, and compare ionic bonds vs covalent bonds. nacl is not a covalent bond. We’ll talk about what is an ionic. In salt, the sodium atom donates its electron, so it yields the. to tell if nacl (table salt) is ionic or covalent (also called molecular) we. . Is Table Salt A Covalent Bond.