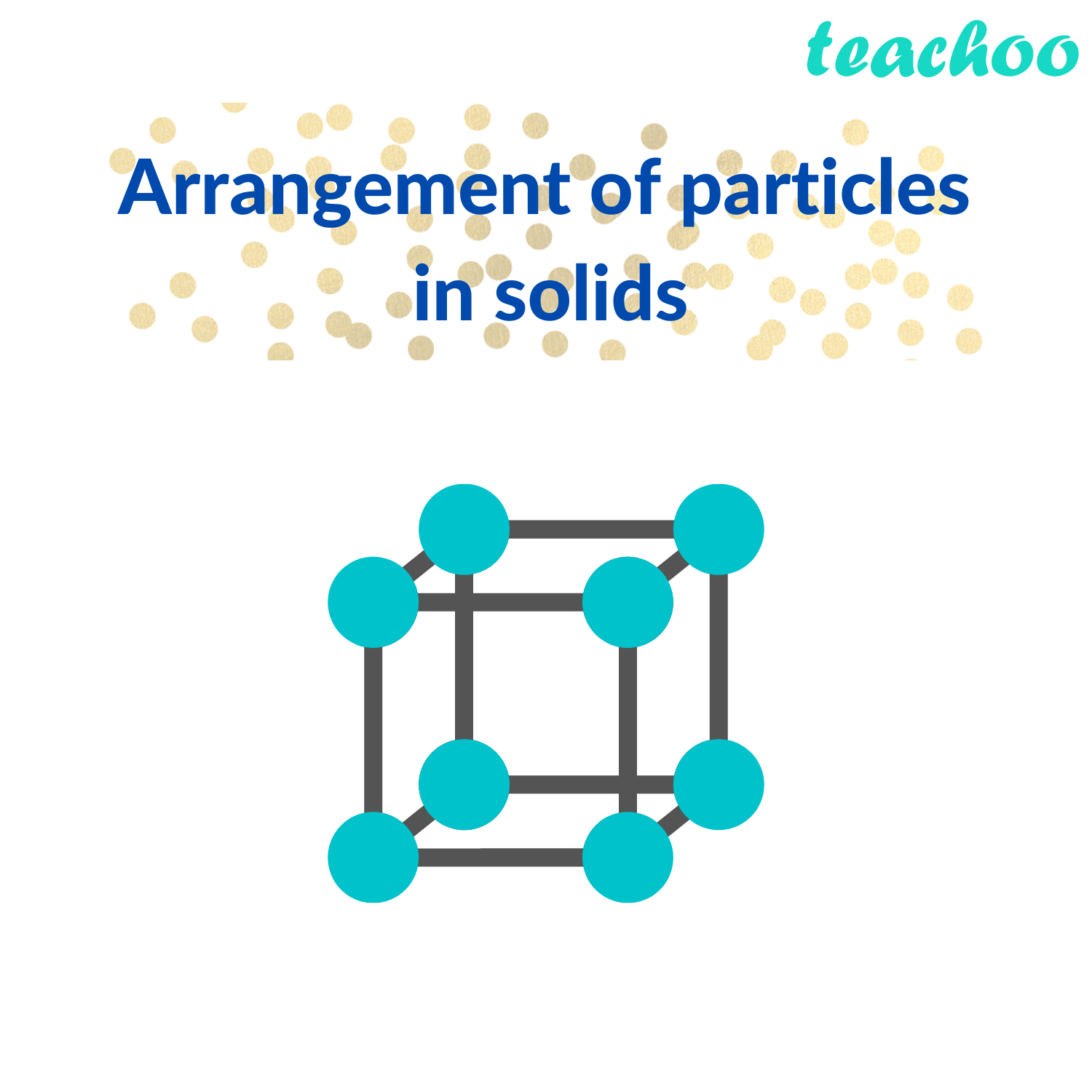
Solids And Liquids Cannot Be Compressed Because . Flow and take the shape of their container, because their particles can move around each other. As a result, solids have a definite shape and volume. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles. Solids such as concrete are useful. They do not fill their entire containers like gases do,. the particles in liquids are arranged in a random way, and are close together, touching many of their. Most solids are hard, but some (like waxes) are. the solid phase has several characteristics. there is no space between the individual particles, so they cannot pack together. the table shows some of the properties of solids and why they are like this: the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. First, solids maintain their shape.
from www.teachoo.com
there is no space between the individual particles, so they cannot pack together. the particles in liquids are arranged in a random way, and are close together, touching many of their. the solid phase has several characteristics. Solids such as concrete are useful. They do not fill their entire containers like gases do,. Flow and take the shape of their container, because their particles can move around each other. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles. First, solids maintain their shape. the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. As a result, solids have a definite shape and volume.
Properties of Solids, Liquids, Gases Compared Teachoo Science
Solids And Liquids Cannot Be Compressed Because the table shows some of the properties of solids and why they are like this: Flow and take the shape of their container, because their particles can move around each other. Solids such as concrete are useful. First, solids maintain their shape. the table shows some of the properties of solids and why they are like this: the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. there is no space between the individual particles, so they cannot pack together. As a result, solids have a definite shape and volume. They do not fill their entire containers like gases do,. Most solids are hard, but some (like waxes) are. the particles in liquids are arranged in a random way, and are close together, touching many of their. the solid phase has several characteristics. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solids And Liquids Cannot Be Compressed Because the table shows some of the properties of solids and why they are like this: As a result, solids have a definite shape and volume. Solids such as concrete are useful. the solid phase has several characteristics. the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot. Solids And Liquids Cannot Be Compressed Because.
From slidetodoc.com
Phases of matter Solids liquids and gases Matter Solids And Liquids Cannot Be Compressed Because Flow and take the shape of their container, because their particles can move around each other. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles. the particles in the solid are tightly. Solids And Liquids Cannot Be Compressed Because.
From slideplayer.com
States of Matter Chapter ppt download Solids And Liquids Cannot Be Compressed Because They do not fill their entire containers like gases do,. Most solids are hard, but some (like waxes) are. the table shows some of the properties of solids and why they are like this: Solids such as concrete are useful. Flow and take the shape of their container, because their particles can move around each other. the particles. Solids And Liquids Cannot Be Compressed Because.
From www.slideserve.com
PPT Solids, Liquids and Gases . PowerPoint Presentation, free Solids And Liquids Cannot Be Compressed Because They do not fill their entire containers like gases do,. Flow and take the shape of their container, because their particles can move around each other. the particles in liquids are arranged in a random way, and are close together, touching many of their. Most solids are hard, but some (like waxes) are. there is no space between. Solids And Liquids Cannot Be Compressed Because.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solids And Liquids Cannot Be Compressed Because Most solids are hard, but some (like waxes) are. the table shows some of the properties of solids and why they are like this: there is no space between the individual particles, so they cannot pack together. First, solids maintain their shape. the solid phase has several characteristics. They do not fill their entire containers like gases. Solids And Liquids Cannot Be Compressed Because.
From www.teachoo.com
Activity to Show Gases can be Compressed but Solids and Liquids cannot Solids And Liquids Cannot Be Compressed Because Flow and take the shape of their container, because their particles can move around each other. the particles in liquids are arranged in a random way, and are close together, touching many of their. there is no space between the individual particles, so they cannot pack together. As a result, solids have a definite shape and volume. . Solids And Liquids Cannot Be Compressed Because.
From byjus.com
Activity To show that solids and liquids cannot be compressed but gases Solids And Liquids Cannot Be Compressed Because First, solids maintain their shape. the solid phase has several characteristics. They do not fill their entire containers like gases do,. As a result, solids have a definite shape and volume. there is no space between the individual particles, so they cannot pack together. Most solids are hard, but some (like waxes) are. solids in the solid. Solids And Liquids Cannot Be Compressed Because.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solids And Liquids Cannot Be Compressed Because They do not fill their entire containers like gases do,. Solids such as concrete are useful. there is no space between the individual particles, so they cannot pack together. the solid phase has several characteristics. the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or. Solids And Liquids Cannot Be Compressed Because.
From slideplayer.com
Particles, solids liquids and gases ppt download Solids And Liquids Cannot Be Compressed Because the solid phase has several characteristics. the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. Solids such as concrete are useful. the table shows some of the properties of solids and why they are like this: Most solids are hard, but some (like. Solids And Liquids Cannot Be Compressed Because.
From www.youtube.com
Can solids, liquids and gases be compressed? Grade Three Science Solids And Liquids Cannot Be Compressed Because the particles in liquids are arranged in a random way, and are close together, touching many of their. As a result, solids have a definite shape and volume. First, solids maintain their shape. there is no space between the individual particles, so they cannot pack together. They do not fill their entire containers like gases do,. the. Solids And Liquids Cannot Be Compressed Because.
From slideplayer.com
Properties of Matter. ppt download Solids And Liquids Cannot Be Compressed Because First, solids maintain their shape. Most solids are hard, but some (like waxes) are. the table shows some of the properties of solids and why they are like this: the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. solids in the solid state,. Solids And Liquids Cannot Be Compressed Because.
From cscyear10chem.weebly.com
Solids, Liquids & Gases Year 10 Chem Solids And Liquids Cannot Be Compressed Because As a result, solids have a definite shape and volume. First, solids maintain their shape. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles. the particles in liquids are arranged in a. Solids And Liquids Cannot Be Compressed Because.
From vitngon24h.com
Can The 3 States Of Matter Be Compressed Exploring The Possibilities Solids And Liquids Cannot Be Compressed Because the table shows some of the properties of solids and why they are like this: the solid phase has several characteristics. First, solids maintain their shape. there is no space between the individual particles, so they cannot pack together. the particles in liquids are arranged in a random way, and are close together, touching many of. Solids And Liquids Cannot Be Compressed Because.
From slideplayer.com
Particles, solids liquids and gases ppt download Solids And Liquids Cannot Be Compressed Because the table shows some of the properties of solids and why they are like this: First, solids maintain their shape. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles. As a result,. Solids And Liquids Cannot Be Compressed Because.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solids And Liquids Cannot Be Compressed Because the particles in liquids are arranged in a random way, and are close together, touching many of their. the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. the solid phase has several characteristics. Most solids are hard, but some (like waxes) are. As. Solids And Liquids Cannot Be Compressed Because.
From slidetodoc.com
States of Matter The Four States of Matter Solids And Liquids Cannot Be Compressed Because the solid phase has several characteristics. Solids such as concrete are useful. First, solids maintain their shape. the table shows some of the properties of solids and why they are like this: there is no space between the individual particles, so they cannot pack together. solids in the solid state, the individual particles of a substance. Solids And Liquids Cannot Be Compressed Because.
From slideplayer.com
Unit 3 Science Investigation Skills ppt download Solids And Liquids Cannot Be Compressed Because the solid phase has several characteristics. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles. Solids such as concrete are useful. They do not fill their entire containers like gases do,. . Solids And Liquids Cannot Be Compressed Because.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Solids And Liquids Cannot Be Compressed Because Solids such as concrete are useful. Most solids are hard, but some (like waxes) are. They do not fill their entire containers like gases do,. there is no space between the individual particles, so they cannot pack together. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other. Solids And Liquids Cannot Be Compressed Because.
From www.britannica.com
solid Definition & Facts Britannica Solids And Liquids Cannot Be Compressed Because They do not fill their entire containers like gases do,. First, solids maintain their shape. the particles in liquids are arranged in a random way, and are close together, touching many of their. Flow and take the shape of their container, because their particles can move around each other. solids in the solid state, the individual particles of. Solids And Liquids Cannot Be Compressed Because.
From slideplayer.com
Density How much stuff. ppt download Solids And Liquids Cannot Be Compressed Because the table shows some of the properties of solids and why they are like this: Flow and take the shape of their container, because their particles can move around each other. there is no space between the individual particles, so they cannot pack together. solids in the solid state, the individual particles of a substance are in. Solids And Liquids Cannot Be Compressed Because.
From sansona.github.io
Solids, Liquids, and Gases Solids And Liquids Cannot Be Compressed Because As a result, solids have a definite shape and volume. Most solids are hard, but some (like waxes) are. First, solids maintain their shape. the table shows some of the properties of solids and why they are like this: there is no space between the individual particles, so they cannot pack together. They do not fill their entire. Solids And Liquids Cannot Be Compressed Because.
From h-o-m-e.org
The Limits of Compression Can Liquids Be Compressed? Solids And Liquids Cannot Be Compressed Because the solid phase has several characteristics. there is no space between the individual particles, so they cannot pack together. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles. the particles. Solids And Liquids Cannot Be Compressed Because.
From slideplayer.com
Particles, solids liquids and gases ppt download Solids And Liquids Cannot Be Compressed Because the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles.. Solids And Liquids Cannot Be Compressed Because.
From slideplayer.com
Matter and Energy. ppt download Solids And Liquids Cannot Be Compressed Because the particles in liquids are arranged in a random way, and are close together, touching many of their. there is no space between the individual particles, so they cannot pack together. First, solids maintain their shape. Solids such as concrete are useful. They do not fill their entire containers like gases do,. As a result, solids have a. Solids And Liquids Cannot Be Compressed Because.
From www.slideserve.com
PPT Solids, Liquids and Gases . PowerPoint Presentation, free Solids And Liquids Cannot Be Compressed Because Most solids are hard, but some (like waxes) are. the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. Solids such as concrete are useful. the table shows some of the properties of solids and why they are like this: there is no space. Solids And Liquids Cannot Be Compressed Because.
From slideplayer.com
Presentation prepared by ppt download Solids And Liquids Cannot Be Compressed Because Most solids are hard, but some (like waxes) are. the particles in liquids are arranged in a random way, and are close together, touching many of their. First, solids maintain their shape. the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. solids in. Solids And Liquids Cannot Be Compressed Because.
From studypositivity.z21.web.core.windows.net
Why Can't Gases Be Compressed Solids And Liquids Cannot Be Compressed Because They do not fill their entire containers like gases do,. Solids such as concrete are useful. the table shows some of the properties of solids and why they are like this: the solid phase has several characteristics. there is no space between the individual particles, so they cannot pack together. the particles in the solid are. Solids And Liquids Cannot Be Compressed Because.
From blog.mensor.com
What Exactly is The Compressibility of Fluids? Solids And Liquids Cannot Be Compressed Because Solids such as concrete are useful. They do not fill their entire containers like gases do,. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles. Flow and take the shape of their container,. Solids And Liquids Cannot Be Compressed Because.
From byjus.com
Why Solids Cannot Be Compressed? Solids And Liquids Cannot Be Compressed Because Most solids are hard, but some (like waxes) are. the solid phase has several characteristics. there is no space between the individual particles, so they cannot pack together. the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. Solids such as concrete are useful.. Solids And Liquids Cannot Be Compressed Because.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solids And Liquids Cannot Be Compressed Because Flow and take the shape of their container, because their particles can move around each other. the table shows some of the properties of solids and why they are like this: Solids such as concrete are useful. the particles in liquids are arranged in a random way, and are close together, touching many of their. They do not. Solids And Liquids Cannot Be Compressed Because.
From slideplayer.com
Unit 4 Lesson 1 What Are Solids, Liquids, and Gases? ppt download Solids And Liquids Cannot Be Compressed Because there is no space between the individual particles, so they cannot pack together. They do not fill their entire containers like gases do,. First, solids maintain their shape. Most solids are hard, but some (like waxes) are. As a result, solids have a definite shape and volume. the solid phase has several characteristics. Solids such as concrete are. Solids And Liquids Cannot Be Compressed Because.
From www.toppr.com
State whether true or falseA liquid cannot be compressed because it Solids And Liquids Cannot Be Compressed Because the table shows some of the properties of solids and why they are like this: As a result, solids have a definite shape and volume. the particles in liquids are arranged in a random way, and are close together, touching many of their. Flow and take the shape of their container, because their particles can move around each. Solids And Liquids Cannot Be Compressed Because.
From keystagewiki.com
Solid Key Stage Wiki Solids And Liquids Cannot Be Compressed Because Flow and take the shape of their container, because their particles can move around each other. First, solids maintain their shape. solids in the solid state, the individual particles of a substance are in fixed positions with respect to each other because there is not enough thermal energy to overcome the intermolecular interactions between the particles. Solids such as. Solids And Liquids Cannot Be Compressed Because.
From slidetodoc.com
Chapter 9 Hydraulics and Pneumatics What we will Solids And Liquids Cannot Be Compressed Because the table shows some of the properties of solids and why they are like this: the solid phase has several characteristics. the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. First, solids maintain their shape. Most solids are hard, but some (like waxes). Solids And Liquids Cannot Be Compressed Because.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solids And Liquids Cannot Be Compressed Because First, solids maintain their shape. As a result, solids have a definite shape and volume. there is no space between the individual particles, so they cannot pack together. Solids such as concrete are useful. the particles in the solid are tightly packed and held in fixed places where they can only vibrate and cannot migrate or flow. Most. Solids And Liquids Cannot Be Compressed Because.