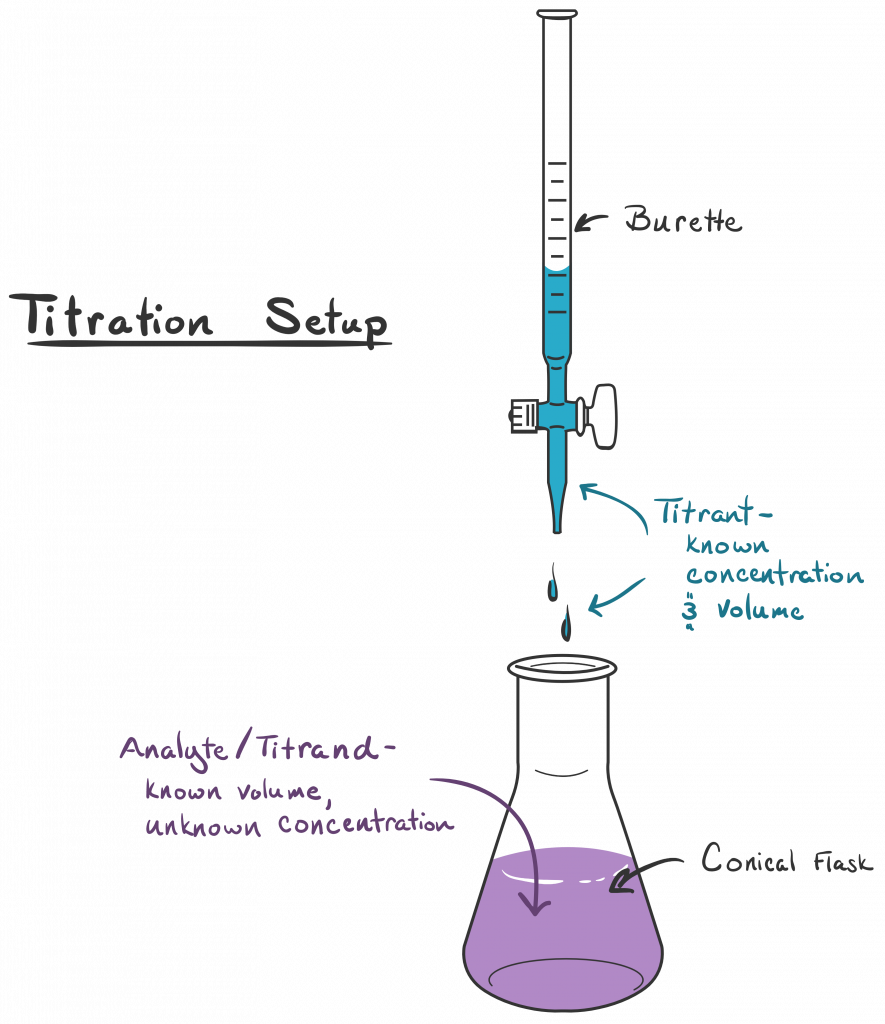
Titration Diagram Labelled . in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. the process involves adding a known solution to the unknown solution until a reaction occurs. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The shape of the graph. the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. Everything you need to know for a level Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. how do you explain the shape of a titration curve? And why is the equivalence point not always at ph7?
from www.microlit.com
the process involves adding a known solution to the unknown solution until a reaction occurs. And why is the equivalence point not always at ph7? the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. Everything you need to know for a level The shape of the graph. how do you explain the shape of a titration curve?
An Advanced Guide to Titration Microlit
Titration Diagram Labelled how do you explain the shape of a titration curve? the process involves adding a known solution to the unknown solution until a reaction occurs. Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. The shape of the graph. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. how do you explain the shape of a titration curve? And why is the equivalence point not always at ph7? Everything you need to know for a level in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Titration Diagram Labelled the process involves adding a known solution to the unknown solution until a reaction occurs. the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. how do you explain the shape of a titration curve? And why is the equivalence point not always at ph7? . Titration Diagram Labelled.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titration Diagram Labelled the process involves adding a known solution to the unknown solution until a reaction occurs. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. And why is the equivalence point not always at ph7? The shape of the graph. in an. Titration Diagram Labelled.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Diagram Labelled during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The shape of the graph. Everything you need to know for a level how do you explain the shape of a titration curve? in an indicator based titration you add another chemical. Titration Diagram Labelled.
From exoomeyij.blob.core.windows.net
Titration Curve Of Amino Acids And Its Significance at Jaclyn Aguilar blog Titration Diagram Labelled And why is the equivalence point not always at ph7? in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. how do you explain the shape of a titration. Titration Diagram Labelled.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Diagram Labelled during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. Everything you need to know for a level Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. the object of a titration is. Titration Diagram Labelled.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Diagram Labelled Everything you need to know for a level in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. the object of a titration is always to add just the. Titration Diagram Labelled.
From www.science-revision.co.uk
Titrations Titration Diagram Labelled And why is the equivalence point not always at ph7? the process involves adding a known solution to the unknown solution until a reaction occurs. Everything you need to know for a level how do you explain the shape of a titration curve? during a titration, ph can be plotted against the volume of acid added to. Titration Diagram Labelled.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Diagram Labelled Everything you need to know for a level the process involves adding a known solution to the unknown solution until a reaction occurs. The shape of the graph. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. how do you explain the shape of a titration. Titration Diagram Labelled.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Diagram Labelled Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. Everything you need to know for a level And why is the equivalence point not always at ph7? the process involves adding a known solution to the unknown solution until a reaction occurs. in an indicator based titration you. Titration Diagram Labelled.
From sbl1023.blogspot.com
LAB 3 TITRATION Titration Diagram Labelled Everything you need to know for a level how do you explain the shape of a titration curve? during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. in an indicator based titration you add another chemical that changes color at the. Titration Diagram Labelled.
From mavink.com
Titration Diagram Labeled Titration Diagram Labelled how do you explain the shape of a titration curve? Everything you need to know for a level The shape of the graph. Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. during a titration, ph can be plotted against the volume of acid added to a basic. Titration Diagram Labelled.
From resource.studiaacademy.com
Acids, Alkalis and Titrations Studia Academy Resources Titration Diagram Labelled in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. Everything you need to know for a level the process involves adding a known solution to the unknown solution. Titration Diagram Labelled.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Diagram Labelled The shape of the graph. the process involves adding a known solution to the unknown solution until a reaction occurs. And why is the equivalence point not always at ph7? during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. Everything you need. Titration Diagram Labelled.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Diagram Labelled Everything you need to know for a level The shape of the graph. the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a. Titration Diagram Labelled.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Diagram Labelled The shape of the graph. Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. And why is the equivalence point not always at ph7? Everything you need to know for a level how do you explain the shape of a titration curve? the process involves adding a known. Titration Diagram Labelled.
From chem.libretexts.org
14.7 AcidBase Titrations Chemistry LibreTexts Titration Diagram Labelled how do you explain the shape of a titration curve? Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. Everything you need to know for a level And why is the equivalence point not always at ph7? The shape of the graph. in an indicator based titration you. Titration Diagram Labelled.
From www.dreamstime.com
Titration Vector Illustration. Labeled Educational Chemistry Process Titration Diagram Labelled how do you explain the shape of a titration curve? The shape of the graph. And why is the equivalence point not always at ph7? during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. the process involves adding a known solution. Titration Diagram Labelled.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Diagram Labelled in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. how do you explain the shape of a titration curve? Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. the object of a titration is always to. Titration Diagram Labelled.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Diagram Labelled in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025. Titration Diagram Labelled.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Diagram Labelled the process involves adding a known solution to the unknown solution until a reaction occurs. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The shape of the graph. the object of a titration is always to add just the amount. Titration Diagram Labelled.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Diagram Labelled the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. in an indicator based titration you add another chemical that changes. Titration Diagram Labelled.
From mavink.com
Titration Labeled Titration Diagram Labelled Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. the process involves adding a known solution to the unknown solution until a reaction occurs. The shape of the graph. And why is the equivalence point not always at ph7? in an indicator based titration you add another chemical. Titration Diagram Labelled.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Diagram Labelled the process involves adding a known solution to the unknown solution until a reaction occurs. Everything you need to know for a level The shape of the graph. the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. And why is the equivalence point not always at. Titration Diagram Labelled.
From school.careers360.com
types of titration Overview, Structure, Properties & Uses Titration Diagram Labelled the process involves adding a known solution to the unknown solution until a reaction occurs. The shape of the graph. Everything you need to know for a level in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. during a titration, ph can be plotted against the. Titration Diagram Labelled.
From bramblechemistry.weebly.com
4C6 Titration Titration Diagram Labelled Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. And why is the equivalence point not always at ph7? during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. in an indicator based. Titration Diagram Labelled.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Diagram Labelled Everything you need to know for a level And why is the equivalence point not always at ph7? during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. the object of a titration is always to add just the amount of titrant needed. Titration Diagram Labelled.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Titration Diagram Labelled The shape of the graph. the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. during a titration, ph can be plotted against the volume of. Titration Diagram Labelled.
From mungfali.com
Titration Curve Labeled Titration Diagram Labelled And why is the equivalence point not always at ph7? in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. the object. Titration Diagram Labelled.
From mavink.com
Titration Labeled Titration Diagram Labelled Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. Everything you need to know for a level during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. in an indicator based titration you. Titration Diagram Labelled.
From www.shutterstock.com
Titration Setup Educational Chemistry Diagram Stock Illustration Titration Diagram Labelled And why is the equivalence point not always at ph7? how do you explain the shape of a titration curve? Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. The shape of the graph. during a titration, ph can be plotted against the volume of acid added to. Titration Diagram Labelled.
From wordwall.net
Titration apparatus Labelled diagram Titration Diagram Labelled The shape of the graph. in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. how do you explain the shape of a titration curve? the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. Work. Titration Diagram Labelled.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Diagram Labelled how do you explain the shape of a titration curve? in an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point,. Everything you need to know for a level the process involves adding a known solution to the unknown solution until a reaction occurs. The shape of the. Titration Diagram Labelled.
From mavink.com
Titration Diagram Labeled Titration Diagram Labelled the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. And why is the equivalence point not always at ph7? during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. how. Titration Diagram Labelled.
From www.youtube.com
Titration tube How to draw well labelled Titration Tube Titration Titration Diagram Labelled Everything you need to know for a level during a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. the object of a titration is always to add just the amount of titrant needed to consume exactly the amount of. in an indicator. Titration Diagram Labelled.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Diagram Labelled Work out amount, in mol, of sodium hydroxide amount = conc x vol = 0.10 x 0.025 = 0. how do you explain the shape of a titration curve? the process involves adding a known solution to the unknown solution until a reaction occurs. during a titration, ph can be plotted against the volume of acid added. Titration Diagram Labelled.