Copper Acetate Reduction Potential . You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. The effect of the acetate counterion appears crucial but has not. However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid.
from www.flinnsci.com
You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical. The effect of the acetate counterion appears crucial but has not. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton.
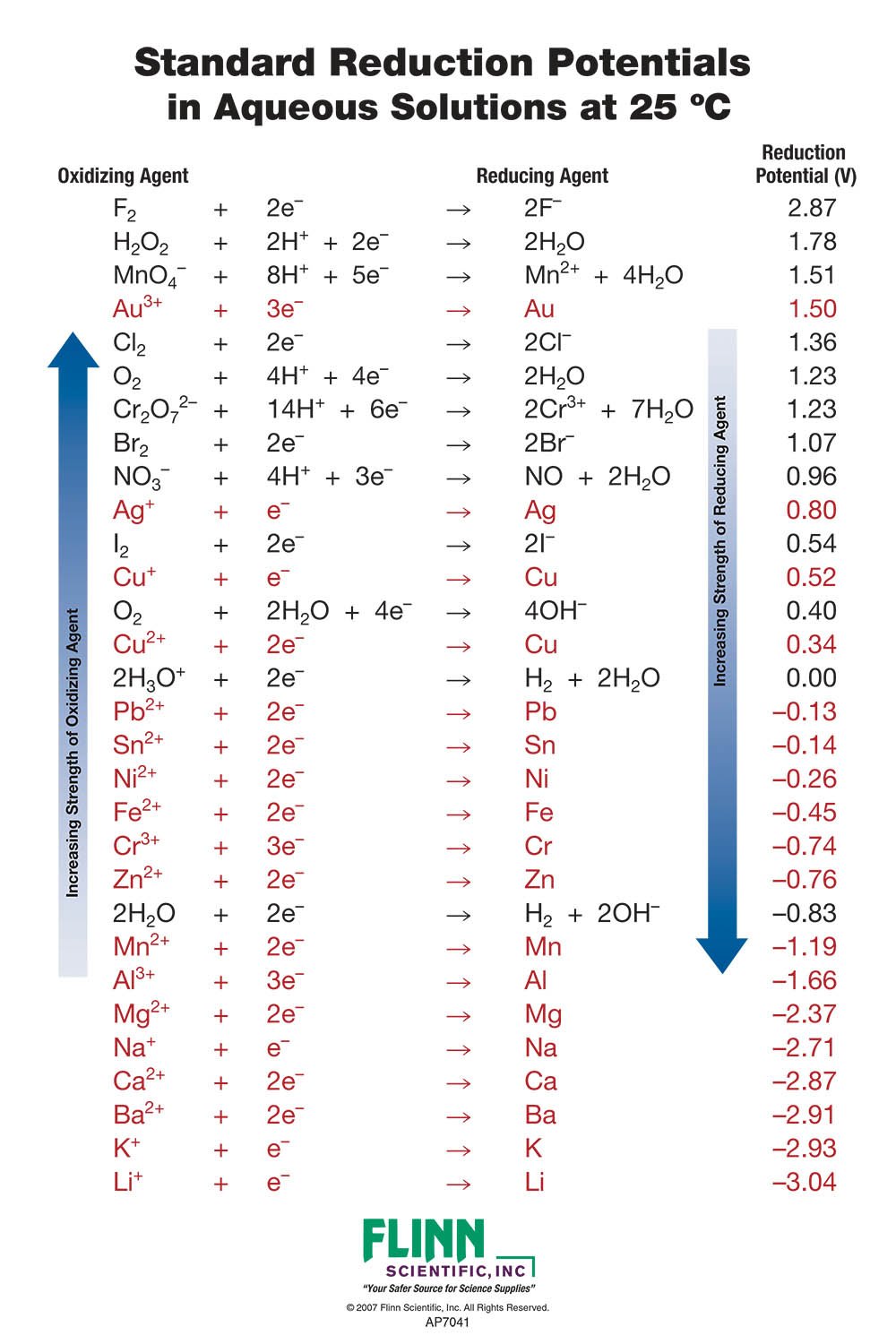
Standard Reduction Potential Chart Flinn Scientific
Copper Acetate Reduction Potential The effect of the acetate counterion appears crucial but has not. The effect of the acetate counterion appears crucial but has not. However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical.
From www.researchgate.net
Comparative diagram of the standard deposition potentials of copper Copper Acetate Reduction Potential However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. The effect of the acetate counterion appears crucial but has not. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. In a 2 m koh electrolyte, the. Copper Acetate Reduction Potential.
From www.researchgate.net
Redox potentials for nickel and copper complexes expressed in V versus Copper Acetate Reduction Potential However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. The effect of the acetate counterion appears crucial but has not. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co). Copper Acetate Reduction Potential.
From pubs.acs.org
PolymerCovered Copper Catalysts Alter the Reaction Pathway of the Copper Acetate Reduction Potential Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of. Copper Acetate Reduction Potential.
From www.researchgate.net
Variation of the opencircuit potential for standard copper Copper Acetate Reduction Potential Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical. The effect of the acetate counterion appears. Copper Acetate Reduction Potential.
From www.youtube.com
Synthesis of Copper Acetate YouTube Copper Acetate Reduction Potential Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol. Copper Acetate Reduction Potential.
From www.chegg.com
Solved 1. Using the table of standard reduction potentials Copper Acetate Reduction Potential You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate. Copper Acetate Reduction Potential.
From www.chegg.com
Solved I. Reduction Potential And Reaction Spontaneity A.... Copper Acetate Reduction Potential Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol. Copper Acetate Reduction Potential.
From pubs.rsc.org
Unexpected high selectivity for acetate formation from CO 2 reduction Copper Acetate Reduction Potential In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical. However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. You may rewrite a reaction by replacing h + with h 3 o. Copper Acetate Reduction Potential.
From www.degruyter.com
Copper ternary oxides as photocathodes for solardriven CO2 reduction Copper Acetate Reduction Potential However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of. Copper Acetate Reduction Potential.
From ch302.cm.utexas.edu
stdpotsshortlist.png Copper Acetate Reduction Potential Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical. However, cu(i) active species are easily reduced to cu(0) during. Copper Acetate Reduction Potential.
From www.researchgate.net
The mechanism for CO reduction on Cu that shows how it splits at Copper Acetate Reduction Potential You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. The effect of the acetate counterion appears crucial but has not. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density. Copper Acetate Reduction Potential.
From www.researchgate.net
Faradaic efficiencies of CO reduction into (a) acetate, (c) ethanol Copper Acetate Reduction Potential However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa),. Copper Acetate Reduction Potential.
From www.chegg.com
Solved Table 5.1Standard Redox Potentials of Selected Copper Acetate Reduction Potential You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate. Copper Acetate Reduction Potential.
From www.researchgate.net
CO2 reduction potentials vs. SHE a . Download Scientific Diagram Copper Acetate Reduction Potential However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical. The effect of the acetate counterion appears crucial but has not. Under anaerobic. Copper Acetate Reduction Potential.
From www.youtube.com
Estimate the standard reduction potential for the copper `//` copper Copper Acetate Reduction Potential Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. The effect of the acetate counterion appears crucial but has not. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in. Copper Acetate Reduction Potential.
From chemrxiv.org
Mechanism for acetate formation in electrochemical CO(2) reduction on Copper Acetate Reduction Potential You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. The effect of the acetate counterion appears crucial but. Copper Acetate Reduction Potential.
From www.youtube.com
Synthesis of Copper Acetate(II) Tutorial and Explanation YouTube Copper Acetate Reduction Potential Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. The effect of the acetate counterion appears crucial but has not. You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. However,. Copper Acetate Reduction Potential.
From www.chem.fsu.edu
Lab Purpose Copper Acetate Reduction Potential You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. The effect of the acetate counterion appears crucial but has not. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. In. Copper Acetate Reduction Potential.
From www.chegg.com
Solved Using the following data calculate the standard Copper Acetate Reduction Potential Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical. However, cu(i) active species are easily reduced to cu(0) during. Copper Acetate Reduction Potential.
From cymitquimica.com
Copper(II) Acetate Monohydrate CymitQuimica Copper Acetate Reduction Potential However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current. Copper Acetate Reduction Potential.
From www.researchgate.net
DFT calculations a, Proposed mechanism for the electroreduction of CO Copper Acetate Reduction Potential The effect of the acetate counterion appears crucial but has not. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. Electroreduction. Copper Acetate Reduction Potential.
From www.researchgate.net
Carbon dioxide reduction over coppercuprous oxide electrodes prepared Copper Acetate Reduction Potential Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. In a 2 m koh electrolyte, the cu nanosheets. Copper Acetate Reduction Potential.
From www.flinnsci.com
Standard Reduction Potential Chart Flinn Scientific Copper Acetate Reduction Potential You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate. Copper Acetate Reduction Potential.
From www.mdpi.com
Catalysts Free FullText Recent Advances in Heterogeneous Copper Acetate Reduction Potential In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical. You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. The effect of the. Copper Acetate Reduction Potential.
From www.researchgate.net
The redox potentials of the photocorrosion reaction of Cu 2 O with Copper Acetate Reduction Potential However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an. Copper Acetate Reduction Potential.
From pubs.rsc.org
Unexpected high selectivity for acetate formation from CO 2 reduction Copper Acetate Reduction Potential The effect of the acetate counterion appears crucial but has not. You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. Electroreduction. Copper Acetate Reduction Potential.
From www.numerade.com
SOLVED write Balanced equation for copper metal and oxygen gas and Copper Acetate Reduction Potential However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. The effect of the acetate counterion appears crucial but has not. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co). Copper Acetate Reduction Potential.
From www.researchgate.net
Cyclic voltammetric response of the metallic copper electrode in 0.1 Copper Acetate Reduction Potential The effect of the acetate counterion appears crucial but has not. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. In a 2 m. Copper Acetate Reduction Potential.
From www.slideserve.com
PPT Organic chemistry ii lab8 PowerPoint Presentation, free download Copper Acetate Reduction Potential The effect of the acetate counterion appears crucial but has not. However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of 48% with an acetate partial current density up to 131 ma cm−2 in electrochemical. You may. Copper Acetate Reduction Potential.
From www.researchgate.net
Redox Potentials of the Complexes Used in the Present Study a Copper Acetate Reduction Potential Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol. Copper Acetate Reduction Potential.
From www.slideserve.com
PPT Knowing Nernst Nonequilibrium copper redox chemistry PowerPoint Copper Acetate Reduction Potential You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. However, cu(i) active species are easily reduced to cu(0). Copper Acetate Reduction Potential.
From www.researchgate.net
Gibbs free energy changes and standard reduction potentials at 25 • C Copper Acetate Reduction Potential You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. The effect of the acetate counterion appears crucial but. Copper Acetate Reduction Potential.
From www.sciencephoto.com
Electron transport chain redox potentials, illustration Stock Image Copper Acetate Reduction Potential Under anaerobic conditions, cu ii x 2 salts [x= acetate (oac), trifluoroacetate (tfa), triflate (otf)] are shown to promote rapid proton. Electroreduction of carbon dioxide (co 2) or carbon monoxide (co) toward c 2+ hydrocarbons such as ethylene, ethanol, acetate and propanol represents a promising. In a 2 m koh electrolyte, the cu nanosheets exhibit an acetate faradaic efficiency of. Copper Acetate Reduction Potential.
From www.mdpi.com
Cellulose AcetateSupported Copper as an Efficient Sustainable Copper Acetate Reduction Potential You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. The effect of the acetate counterion appears crucial but has not. However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. Electroreduction of carbon dioxide. Copper Acetate Reduction Potential.
From www.researchgate.net
Carbon dioxide reduction over various copperbased electrocatalysts. a Copper Acetate Reduction Potential The effect of the acetate counterion appears crucial but has not. However, cu(i) active species are easily reduced to cu(0) during the co 2 rr, leading to a rapid. You may rewrite a reaction by replacing h + with h 3 o + and adding to the opposite side of the reaction one molecule of h. Electroreduction of carbon dioxide. Copper Acetate Reduction Potential.