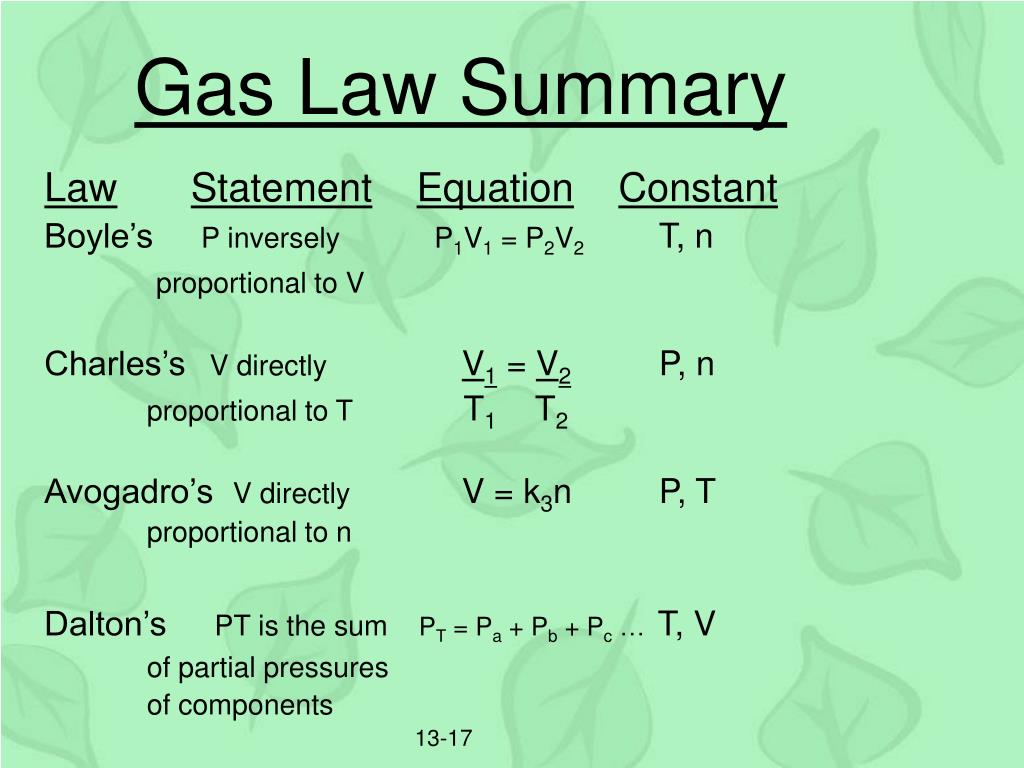
Gas Law Summary . Gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Learn their types, statements, and formula. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Also, learn how to do and solve gas law problems. What are the gas laws.
from www.slideserve.com
Also, learn how to do and solve gas law problems. What are the gas laws. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Learn their types, statements, and formula. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies.
PPT Chapter 13 Gases PowerPoint Presentation, free download ID464161
Gas Law Summary Learn their types, statements, and formula. Also, learn how to do and solve gas law problems. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Learn their types, statements, and formula. What are the gas laws. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Gas Law Summary Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between. Gas Law Summary.
From studylib.net
ABCD Gas Laws summary Gas Law Summary Also, learn how to do and solve gas law problems. What are the gas laws. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Learn their types, statements, and formula. The pressure, volume, and temperature of. Gas Law Summary.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID5614600 Gas Law Summary Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Also, learn how to do and solve gas law problems. What are the gas laws. Ideal gas law, relation between the. Gas Law Summary.
From printablelistgather.z4.web.core.windows.net
Gas Law Formulas Sheet Gas Law Summary Gas laws, laws that relate the pressure, volume, and temperature of a gas. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. What are the gas laws. Learn their types, statements, and formula. The pressure, volume,. Gas Law Summary.
From learncheme.com
idealgaslawsummary LearnChemE Gas Law Summary Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. What are the gas laws. Learn their types, statements,. Gas Law Summary.
From www.slideserve.com
PPT Gases Key Points PowerPoint Presentation, free download ID3846981 Gas Law Summary Gas laws, laws that relate the pressure, volume, and temperature of a gas. What are the gas laws. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Learn their types, statements, and formula. Boyle’s law describes. Gas Law Summary.
From www.slideserve.com
PPT How Do Gases Behave? PowerPoint Presentation, free download ID Gas Law Summary Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Also, learn how to do and solve gas law problems. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a. Gas Law Summary.
From www.slideserve.com
PPT Chapter 13 Gases PowerPoint Presentation, free download ID464161 Gas Law Summary Gas laws, laws that relate the pressure, volume, and temperature of a gas. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Also, learn how to do and solve gas law problems. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas law,. Gas Law Summary.
From www.slideserve.com
PPT Gases Key Points PowerPoint Presentation, free download ID3846981 Gas Law Summary The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Learn their types, statements, and formula. Also, learn how to do and solve gas law problems. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Ideal gas law, relation between the pressure. Gas Law Summary.
From www.slideserve.com
PPT Gas Laws and Nature of Gases PowerPoint Presentation ID5702876 Gas Law Summary Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Boyle’s law describes the inverse proportional relationship between pressure and volume. Gas Law Summary.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID4712528 Gas Law Summary The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Learn their types, statements, and formula. Gas laws, laws that relate the pressure, volume, and temperature of a gas. The pressure, volume, and temperature of most. Gas Law Summary.
From www.slideserve.com
PPT CHAPTER 12 GASES AND THEORY PowerPoint Gas Law Summary Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Gas laws, laws that relate the pressure, volume, and temperature of a gas. What are the gas laws. Also, learn how to do and solve gas law problems. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount. Gas Law Summary.
From www.slideserve.com
PPT Properties of Gases & The gas Laws PowerPoint Presentation ID Gas Law Summary Also, learn how to do and solve gas law problems. What are the gas laws. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The pressure, volume, and temperature of most gases can. Gas Law Summary.
From general.chemistrysteps.com
The Ideal Gas Law Chemistry Steps Gas Law Summary What are the gas laws. Learn their types, statements, and formula. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the. Gas Law Summary.
From www.slideserve.com
PPT Chapter 6 Properties of Gases The Air We Breathe PowerPoint Gas Law Summary The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Also, learn how to do and solve gas law problems. Learn their types, statements, and formula. Boyle’s law describes the inverse proportional relationship. Gas Law Summary.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube Gas Law Summary What are the gas laws. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Also, learn how to do and solve gas law problems. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high. Gas Law Summary.
From www.slideserve.com
PPT Properties of Gases & The gas Laws PowerPoint Presentation ID Gas Law Summary Learn their types, statements, and formula. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The pressure, volume, and temperature. Gas Law Summary.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Gas Law Summary The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law describes the inverse proportional relationship between. Gas Law Summary.
From oertx.highered.texas.gov
General Chemistry for Science Majors, Unit 3, Gas Laws OERTX Gas Law Summary The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Gas laws, laws that relate the pressure, volume, and temperature of a gas. What are the gas laws. Learn their types, statements, and formula. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of. Gas Law Summary.
From www.slideserve.com
PPT Gases Key Points PowerPoint Presentation, free download ID3846981 Gas Law Summary The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Also, learn how to do and solve gas law problems. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Gas laws, laws that relate the pressure, volume, and temperature of a gas.. Gas Law Summary.
From www.studypool.com
SOLUTION Gas law summary Studypool Gas Law Summary Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Boyle’s law —named for robert boyle —states that, at. Gas Law Summary.
From www.slideserve.com
PPT Summary of Gas Laws PowerPoint Presentation, free download ID Gas Law Summary The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. What are the gas laws. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Also, learn how to do and. Gas Law Summary.
From mungfali.com
Gas Laws Formula Sheet Gas Law Summary Learn their types, statements, and formula. Also, learn how to do and solve gas law problems. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Gas laws, laws that relate the pressure, volume, and temperature of. Gas Law Summary.
From www.thinkswap.com
Gas Laws explanations Chemistry Year 11 HSC Thinkswap Gas Law Summary Learn their types, statements, and formula. Also, learn how to do and solve gas law problems. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and. Gas Law Summary.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID6400259 Gas Law Summary Gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules. Gas Law Summary.
From tomi.digital
TOMi.digital Gas Laws Part II Gas Law Summary Gas laws, laws that relate the pressure, volume, and temperature of a gas. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ideal gas law, relation between the pressure p, volume v, and temperature t. Gas Law Summary.
From scienceducatio.weebly.com
Gas Laws Environmental Chemistry Gas Law Summary Learn their types, statements, and formula. What are the gas laws. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Boyle’s law describes the inverse proportional relationship between pressure and volume at. Gas Law Summary.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID6203540 Gas Law Summary Learn their types, statements, and formula. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The three fundamental gas laws discover the relationship. Gas Law Summary.
From studylib.net
Gas Laws Summary Handout Gas Law Summary The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Also, learn how to do and solve gas law problems. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Learn. Gas Law Summary.
From www.studypool.com
SOLUTION Gas law summary chart Studypool Gas Law Summary Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Learn their types, statements, and formula. Also, learn how to do and solve gas law problems. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Gas laws, laws that relate the pressure, volume, and temperature. Gas Law Summary.
From www.slideserve.com
PPT Gases Key Points PowerPoint Presentation, free download ID3846981 Gas Law Summary Learn their types, statements, and formula. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. What are the gas laws. Also, learn how to do and solve gas law problems. Gas laws, laws that relate the pressure, volume, and temperature of a gas. The three fundamental gas laws. Gas Law Summary.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID2984680 Gas Law Summary The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Also, learn how to do and solve gas. Gas Law Summary.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Gas Law Summary Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The pressure, volume, and temperature of most gases can. Gas Law Summary.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID7010709 Gas Law Summary Learn their types, statements, and formula. What are the gas laws. The pressure, volume, and temperature of most gases can be described with simple mathematical relationships that are summarized in one ideal. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the. Gas Law Summary.
From www.slideserve.com
PPT Gases & Atmospheric Chemistry PowerPoint Presentation, free Gas Law Summary Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Also, learn how to do and solve gas law problems. Learn their types, statements, and formula. Ideal gas law, relation between the pressure p,. Gas Law Summary.