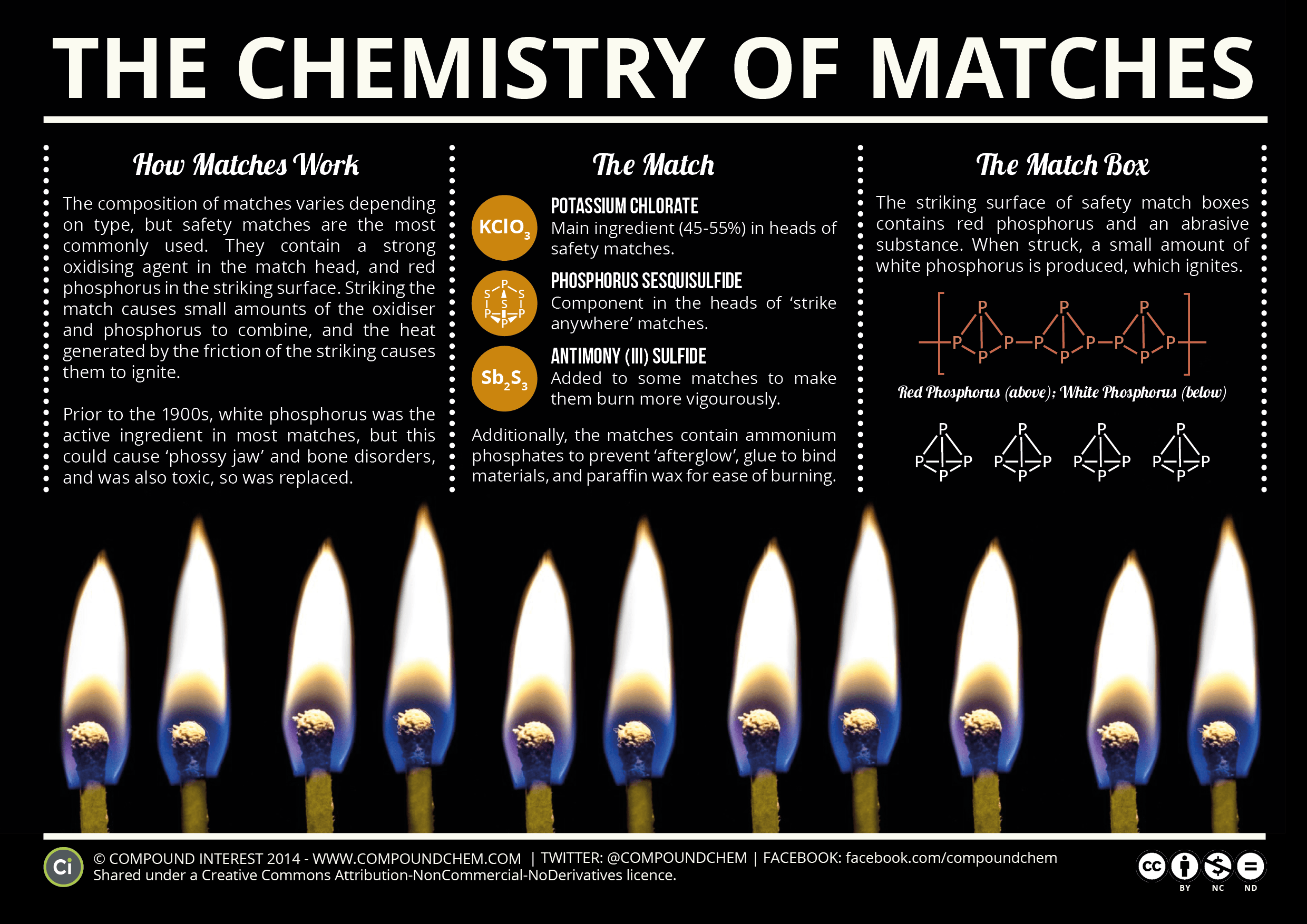
When A Match Is Lit What Energy Converts To What And What Energy . The head of a match uses antimony trisulfide for fuel. After being lit, the match releases. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. Today's matches create fire as the result of a simple chemical reaction. (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. Before being lit, a match has potential energy stored in the chemical bonds of the match head and stick. Potassium chlorate helps that fuel burn and is basically the key to ignition,. You rub the match head against the red. When a match is struck, friction creates heat and a flammable compound that ignites in the air. If the match is struck against the striking surface, the friction. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion.
from www.compoundchem.com
Before being lit, a match has potential energy stored in the chemical bonds of the match head and stick. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. Potassium chlorate helps that fuel burn and is basically the key to ignition,. Today's matches create fire as the result of a simple chemical reaction. (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. The head of a match uses antimony trisulfide for fuel. If the match is struck against the striking surface, the friction. You rub the match head against the red. When a match is struck, friction creates heat and a flammable compound that ignites in the air.
The Chemistry of Matches Compound Interest
When A Match Is Lit What Energy Converts To What And What Energy After being lit, the match releases. The head of a match uses antimony trisulfide for fuel. Today's matches create fire as the result of a simple chemical reaction. After being lit, the match releases. (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. Before being lit, a match has potential energy stored in the chemical bonds of the match head and stick. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. You rub the match head against the red. Potassium chlorate helps that fuel burn and is basically the key to ignition,. If the match is struck against the striking surface, the friction. When a match is struck, friction creates heat and a flammable compound that ignites in the air.
From www.compoundchem.com
The Chemistry of Matches Compound Interest When A Match Is Lit What Energy Converts To What And What Energy If the match is struck against the striking surface, the friction. You rub the match head against the red. Today's matches create fire as the result of a simple chemical reaction. Potassium chlorate helps that fuel burn and is basically the key to ignition,. After being lit, the match releases. When a match is struck, friction creates heat and a. When A Match Is Lit What Energy Converts To What And What Energy.
From www.slideserve.com
PPT Energy Transformation PowerPoint Presentation, free download ID When A Match Is Lit What Energy Converts To What And What Energy Before being lit, a match has potential energy stored in the chemical bonds of the match head and stick. Potassium chlorate helps that fuel burn and is basically the key to ignition,. The head of a match uses antimony trisulfide for fuel. If the match is struck against the striking surface, the friction. When a match is struck, friction creates. When A Match Is Lit What Energy Converts To What And What Energy.
From www.chegg.com
Solved The following is a diagram of energy states and When A Match Is Lit What Energy Converts To What And What Energy You rub the match head against the red. When a match is struck, friction creates heat and a flammable compound that ignites in the air. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. Today's matches create fire as the result of a simple chemical reaction. Before being lit, a match has potential energy stored in. When A Match Is Lit What Energy Converts To What And What Energy.
From brainly.ph
A BC What's More Activity 1 Forms of Energy Match the form of energy When A Match Is Lit What Energy Converts To What And What Energy You rub the match head against the red. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. If the match is struck against the striking surface, the friction. (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. Potassium chlorate helps that fuel burn. When A Match Is Lit What Energy Converts To What And What Energy.
From studylib.net
Energy Transformations Practice When A Match Is Lit What Energy Converts To What And What Energy Potassium chlorate helps that fuel burn and is basically the key to ignition,. The head of a match uses antimony trisulfide for fuel. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. After being lit, the match releases. You rub the match head against the red. (for the adults reading, friction converts kinetic energy into thermal. When A Match Is Lit What Energy Converts To What And What Energy.
From brainly.com
Match each energy type with the source uses When A Match Is Lit What Energy Converts To What And What Energy After being lit, the match releases. (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. Today's matches create fire as the result of a simple chemical reaction. When a match is struck, friction creates heat and a flammable compound that ignites in the air. You rub the. When A Match Is Lit What Energy Converts To What And What Energy.
From quizlet.com
Energy Transformation example Diagram Quizlet When A Match Is Lit What Energy Converts To What And What Energy After being lit, the match releases. Potassium chlorate helps that fuel burn and is basically the key to ignition,. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. The head of a match uses antimony trisulfide for fuel. Today's matches create fire as the result of. When A Match Is Lit What Energy Converts To What And What Energy.
From scienceislife4sgss.weebly.com
Conservation of Energy SCIENCE IS LIFE When A Match Is Lit What Energy Converts To What And What Energy The head of a match uses antimony trisulfide for fuel. Before being lit, a match has potential energy stored in the chemical bonds of the match head and stick. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. After being lit, the match releases. When a. When A Match Is Lit What Energy Converts To What And What Energy.
From www.chegg.com
Solved Match the correct types of energy to the scenario When A Match Is Lit What Energy Converts To What And What Energy (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. After being lit, the match releases. Production of energy (light) lighting a matchstick involves a. When A Match Is Lit What Energy Converts To What And What Energy.
From www.thoughtco.com
Activation Energy (Ea) Chemistry Definition When A Match Is Lit What Energy Converts To What And What Energy Before being lit, a match has potential energy stored in the chemical bonds of the match head and stick. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. The head of a match uses antimony trisulfide for fuel. When you light a match, chemical potential energy stored in the match head is converted into thermal energy. When A Match Is Lit What Energy Converts To What And What Energy.
From www.studypool.com
SOLUTION Energy conversions and forms Studypool When A Match Is Lit What Energy Converts To What And What Energy Today's matches create fire as the result of a simple chemical reaction. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. Potassium chlorate helps that fuel burn and is basically the key to. When A Match Is Lit What Energy Converts To What And What Energy.
From www.studypool.com
SOLUTION Energy conversions and forms Studypool When A Match Is Lit What Energy Converts To What And What Energy If the match is struck against the striking surface, the friction. When a match is struck, friction creates heat and a flammable compound that ignites in the air. Today's matches create fire as the result of a simple chemical reaction. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. When you light a match, chemical potential. When A Match Is Lit What Energy Converts To What And What Energy.
From www.chegg.com
Solved The following is a diagram of energy states and When A Match Is Lit What Energy Converts To What And What Energy Today's matches create fire as the result of a simple chemical reaction. If the match is struck against the striking surface, the friction. You rub the match head against the red. Potassium chlorate helps that fuel burn and is basically the key to ignition,. Before being lit, a match has potential energy stored in the chemical bonds of the match. When A Match Is Lit What Energy Converts To What And What Energy.
From www.slideserve.com
PPT Energy Transformation PowerPoint Presentation, free download ID When A Match Is Lit What Energy Converts To What And What Energy The head of a match uses antimony trisulfide for fuel. Today's matches create fire as the result of a simple chemical reaction. You rub the match head against the red. After being lit, the match releases. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. When a match is struck, friction creates heat and a flammable. When A Match Is Lit What Energy Converts To What And What Energy.
From brainly.com
Organisms convert chemical energy into other types of of energy in When A Match Is Lit What Energy Converts To What And What Energy If the match is struck against the striking surface, the friction. Potassium chlorate helps that fuel burn and is basically the key to ignition,. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. When a match is struck, friction creates heat and a flammable compound that. When A Match Is Lit What Energy Converts To What And What Energy.
From www.chegg.com
Solved Part A Label the energy diagram by matching each term When A Match Is Lit What Energy Converts To What And What Energy Potassium chlorate helps that fuel burn and is basically the key to ignition,. If the match is struck against the striking surface, the friction. When a match is struck, friction creates heat and a flammable compound that ignites in the air. Today's matches create fire as the result of a simple chemical reaction. When you light a match, chemical potential. When A Match Is Lit What Energy Converts To What And What Energy.
From brainly.com
2 Drag the tiles to the correct boxes to complete the pairs. Match each When A Match Is Lit What Energy Converts To What And What Energy After being lit, the match releases. You rub the match head against the red. When a match is struck, friction creates heat and a flammable compound that ignites in the air. Potassium chlorate helps that fuel burn and is basically the key to ignition,. The head of a match uses antimony trisulfide for fuel. (for the adults reading, friction converts. When A Match Is Lit What Energy Converts To What And What Energy.
From brainly.com
Match each form of energy to it's description Motion energy Thermal When A Match Is Lit What Energy Converts To What And What Energy (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. When a match is struck, friction creates heat and a flammable compound that ignites in the air. After being lit, the match releases. When you light a match, chemical potential energy stored in the match head is converted. When A Match Is Lit What Energy Converts To What And What Energy.
From quizizz.com
Energy stores and transfers AQA GCSE Physics Quizizz When A Match Is Lit What Energy Converts To What And What Energy Potassium chlorate helps that fuel burn and is basically the key to ignition,. You rub the match head against the red. After being lit, the match releases. Today's matches create fire as the result of a simple chemical reaction. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form. When A Match Is Lit What Energy Converts To What And What Energy.
From brainly.com
Match each form of energy with its description. gravitational potential When A Match Is Lit What Energy Converts To What And What Energy If the match is struck against the striking surface, the friction. The head of a match uses antimony trisulfide for fuel. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. Today's matches create fire as the result of a simple chemical reaction. Potassium chlorate helps that. When A Match Is Lit What Energy Converts To What And What Energy.
From brainly.com
Match each phrase to the form of energy it represents. chemical When A Match Is Lit What Energy Converts To What And What Energy (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. You rub the match head against the red. When a match is struck, friction creates heat and a flammable compound that ignites in the air. If the match is struck against the striking surface, the friction. The head. When A Match Is Lit What Energy Converts To What And What Energy.
From gustavogargiulo.com
12 Electron Energy And Light Answer Key GustavoGargiulo free When A Match Is Lit What Energy Converts To What And What Energy Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. You rub the match head against the red. Potassium chlorate helps that fuel burn and is basically the key to ignition,. (for the adults. When A Match Is Lit What Energy Converts To What And What Energy.
From murrayscience6.weebly.com
Energy Types and Forms Miss Murray's Science Site When A Match Is Lit What Energy Converts To What And What Energy Before being lit, a match has potential energy stored in the chemical bonds of the match head and stick. (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. Today's matches create fire as the result of a simple chemical reaction. The head of a match uses antimony. When A Match Is Lit What Energy Converts To What And What Energy.
From geomodderfied.weebly.com
Forms of Energy GEOMODDERFIED When A Match Is Lit What Energy Converts To What And What Energy If the match is struck against the striking surface, the friction. When a match is struck, friction creates heat and a flammable compound that ignites in the air. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. Today's matches create fire as the result of a simple chemical reaction. Potassium chlorate helps that fuel burn and. When A Match Is Lit What Energy Converts To What And What Energy.
From scienceislife4sgss.weebly.com
Conservation of Energy SCIENCE IS LIFE When A Match Is Lit What Energy Converts To What And What Energy (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. If the match is struck against the striking surface, the friction. Potassium chlorate helps that fuel burn and is basically the key to ignition,. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. The. When A Match Is Lit What Energy Converts To What And What Energy.
From brainly.com
Match the process in electricity generation to the type of energy When A Match Is Lit What Energy Converts To What And What Energy The head of a match uses antimony trisulfide for fuel. (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. When a match is struck,. When A Match Is Lit What Energy Converts To What And What Energy.
From www.chegg.com
Solved The following is a diagram of energy states and When A Match Is Lit What Energy Converts To What And What Energy The head of a match uses antimony trisulfide for fuel. Today's matches create fire as the result of a simple chemical reaction. If the match is struck against the striking surface, the friction. Potassium chlorate helps that fuel burn and is basically the key to ignition,. When you light a match, chemical potential energy stored in the match head is. When A Match Is Lit What Energy Converts To What And What Energy.
From www.chegg.com
Solved Match each energy type with its definition. Energy When A Match Is Lit What Energy Converts To What And What Energy The head of a match uses antimony trisulfide for fuel. When a match is struck, friction creates heat and a flammable compound that ignites in the air. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the. When A Match Is Lit What Energy Converts To What And What Energy.
From www.ehow.com
The Energy Transformation of a Match Sciencing When A Match Is Lit What Energy Converts To What And What Energy When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. Today's matches create fire as the result of a simple chemical reaction. Before being lit, a match has potential energy stored in the chemical bonds of the match head and stick. Production of energy (light) lighting a. When A Match Is Lit What Energy Converts To What And What Energy.
From towdahaudio.blogspot.com
Towdah tō·dä' Energy Forms, Motion, Heat, Light, Sound, Electricity... When A Match Is Lit What Energy Converts To What And What Energy (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. When a match is struck, friction creates heat and a flammable compound that ignites in the air. The head of a match uses antimony trisulfide for fuel. Production of energy (light) lighting a matchstick involves a chemical reaction. When A Match Is Lit What Energy Converts To What And What Energy.
From www.slideserve.com
PPT Energy Conversion PowerPoint Presentation, free download ID9379829 When A Match Is Lit What Energy Converts To What And What Energy Before being lit, a match has potential energy stored in the chemical bonds of the match head and stick. The head of a match uses antimony trisulfide for fuel. Potassium chlorate helps that fuel burn and is basically the key to ignition,. Production of energy (light) lighting a matchstick involves a chemical reaction called combustion. You rub the match head. When A Match Is Lit What Energy Converts To What And What Energy.
From studylib.net
Energy Worksheet When A Match Is Lit What Energy Converts To What And What Energy After being lit, the match releases. The head of a match uses antimony trisulfide for fuel. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a. When A Match Is Lit What Energy Converts To What And What Energy.
From mylafershiggins.blogspot.com
Match Each Scenario to the Energy Transformation It Represents When A Match Is Lit What Energy Converts To What And What Energy When a match is struck, friction creates heat and a flammable compound that ignites in the air. Today's matches create fire as the result of a simple chemical reaction. The head of a match uses antimony trisulfide for fuel. You rub the match head against the red. Potassium chlorate helps that fuel burn and is basically the key to ignition,.. When A Match Is Lit What Energy Converts To What And What Energy.
From fyooitvgz.blob.core.windows.net
What Form Of Energy Does A Match Have Before It Is Lit at Tonie When A Match Is Lit What Energy Converts To What And What Energy When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. You rub the match head against the red. (for the adults reading, friction converts kinetic energy into thermal energy.) friction is important for the first part of lighting a match. Potassium chlorate helps that fuel burn and. When A Match Is Lit What Energy Converts To What And What Energy.
From www.sciencefacts.net
Energy Transformation (Conversion) Definition and Examples When A Match Is Lit What Energy Converts To What And What Energy Before being lit, a match has potential energy stored in the chemical bonds of the match head and stick. The head of a match uses antimony trisulfide for fuel. When you light a match, chemical potential energy stored in the match head is converted into thermal energy in the form of a. Today's matches create fire as the result of. When A Match Is Lit What Energy Converts To What And What Energy.