Catalyst Reaction Examples Class 10 . Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. A catalyst refers to a substance that can speed up a chemical reaction. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. The reaction which involves a catalyst in their. Learn what catalyst is in detail, types of catalyst along with important. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are substances which alter the rate of reaction by changing the path of reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work.
from www.mdpi.com
This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. The reaction which involves a catalyst in their. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst refers to a substance that can speed up a chemical reaction. Learn what catalyst is in detail, types of catalyst along with important. Catalysts are substances which alter the rate of reaction by changing the path of reaction.
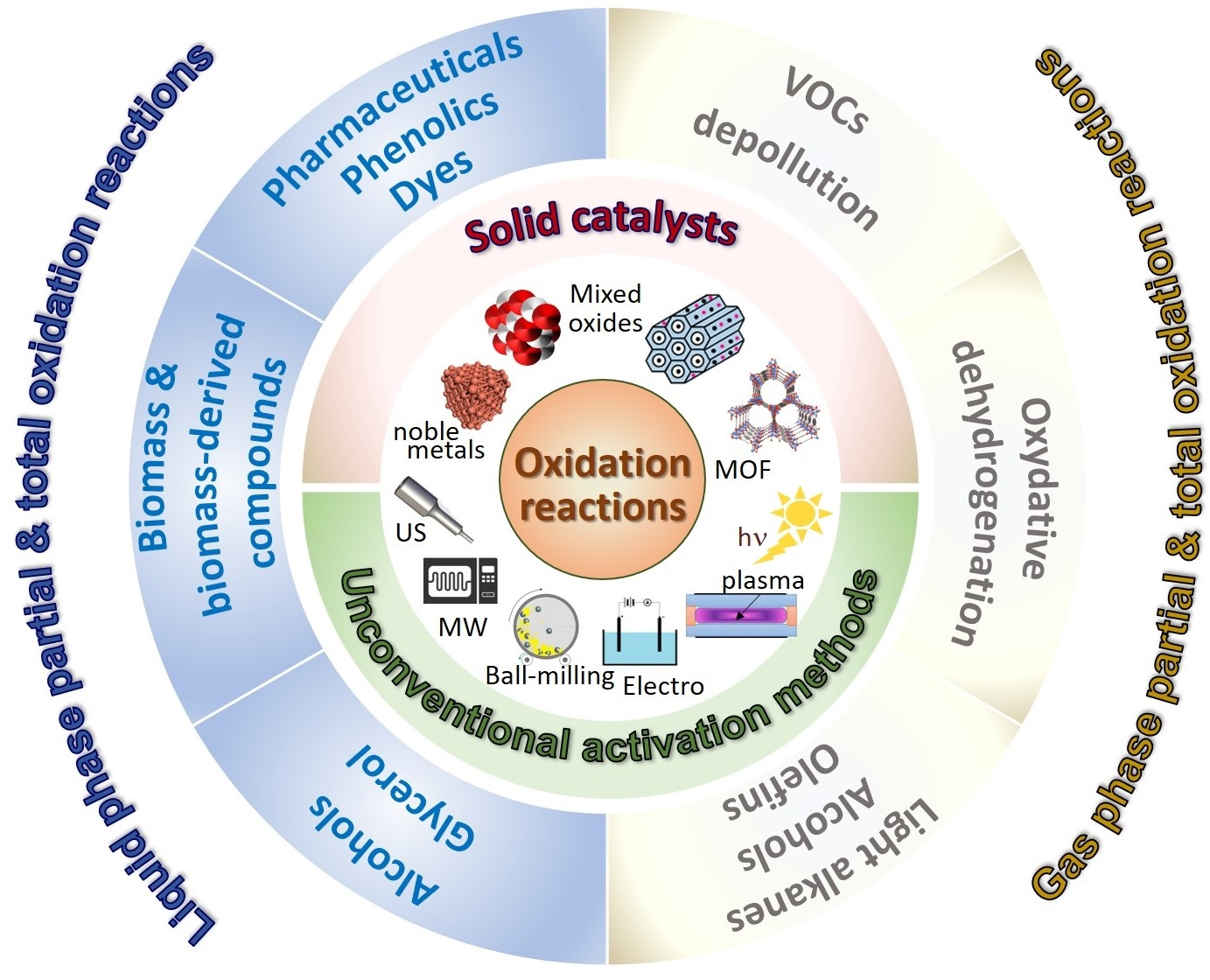
Catalysts Free FullText General and Prospective Views on Oxidation
Catalyst Reaction Examples Class 10 The reaction which involves a catalyst in their. The reaction which involves a catalyst in their. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Learn what catalyst is in detail, types of catalyst along with important. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. Catalysts are substances which alter the rate of reaction by changing the path of reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. A catalyst refers to a substance that can speed up a chemical reaction.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Reaction Examples Class 10 This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst refers to a substance that can speed up a chemical reaction. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. Catalysts are substances. Catalyst Reaction Examples Class 10.
From brainly.in
define catalyst with example ?? Brainly.in Catalyst Reaction Examples Class 10 Learn what catalyst is in detail, types of catalyst along with important. A catalyst refers to a substance that can speed up a chemical reaction. Catalysts are substances which alter the rate of reaction by changing the path of reaction. The reaction which involves a catalyst in their. This page looks at the the different types of catalyst (heterogeneous and. Catalyst Reaction Examples Class 10.
From saylordotorg.github.io
Catalysis Catalyst Reaction Examples Class 10 This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by. Catalyst Reaction Examples Class 10.
From exohjoafd.blob.core.windows.net
Catalyst That Is Not Protein at Timothy Lafortune blog Catalyst Reaction Examples Class 10 Learn what catalyst is in detail, types of catalyst along with important. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. Catalysts are substances which alter the. Catalyst Reaction Examples Class 10.
From www.mdpi.com
Catalysts Free FullText Unprecedented Multifunctionality of Grubbs Catalyst Reaction Examples Class 10 Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst refers to a substance that can speed up a chemical reaction. Learn what catalyst is in detail, types of catalyst along with important. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed. Catalyst Reaction Examples Class 10.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Reaction Examples Class 10 The reaction which involves a catalyst in their. Learn what catalyst is in detail, types of catalyst along with important. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the. Catalyst Reaction Examples Class 10.
From www.youtube.com
Effect of Various Catalysts on Hydrogen Peroxide GCSE Chemistry YouTube Catalyst Reaction Examples Class 10 A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. The reaction which involves a catalyst in their. Catalyst, in chemistry, any substance that increases the rate of. Catalyst Reaction Examples Class 10.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Reaction Examples Class 10 Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn what catalyst is in detail, types of catalyst along with important. Catalysts are substances which alter the rate of reaction by changing the path of reaction. A catalyst refers to a substance that can speed up a chemical reaction. Catalysts are those substances. Catalyst Reaction Examples Class 10.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Reaction Examples Class 10 Learn what catalyst is in detail, types of catalyst along with important. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. A catalyst refers to a substance. Catalyst Reaction Examples Class 10.
From www.youtube.com
Chemical Reactions and Equations Mind Map Class 10 YouTube Catalyst Reaction Examples Class 10 The reaction which involves a catalyst in their. A catalyst refers to a substance that can speed up a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn what catalyst is in detail, types of catalyst along with important. Catalysts are those substances which increase or decrease the rate of. Catalyst Reaction Examples Class 10.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Reaction Examples Class 10 Learn what catalyst is in detail, types of catalyst along with important. The reaction which involves a catalyst in their. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed. Catalyst Reaction Examples Class 10.
From www.mdpi.com
Catalysts Free FullText AirStable Efficient Nickel Catalyst for Catalyst Reaction Examples Class 10 This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. The reaction which involves a catalyst in their. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. Catalysts are substances which alter the rate of. Catalyst Reaction Examples Class 10.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube Catalyst Reaction Examples Class 10 This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst refers to a substance that can speed up a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are substances which alter the rate. Catalyst Reaction Examples Class 10.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Reaction Examples Class 10 A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. A catalyst refers to a substance that can speed up a chemical reaction. Catalysts are substances which alter the rate of reaction by changing the path of reaction. This page looks at the the different types of catalyst. Catalyst Reaction Examples Class 10.
From fyopmikiw.blob.core.windows.net
Catalyst Definition For Class 10 at Mary Clevenger blog Catalyst Reaction Examples Class 10 Learn what catalyst is in detail, types of catalyst along with important. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in. Catalyst Reaction Examples Class 10.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Catalyst Reaction Examples Class 10 Learn what catalyst is in detail, types of catalyst along with important. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst refers to a substance that can. Catalyst Reaction Examples Class 10.
From www.mdpi.com
Catalysts Free FullText Recovery/Reuse of Heterogeneous Supported Catalyst Reaction Examples Class 10 Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst refers to a substance that can speed up a chemical reaction. Learn what catalyst. Catalyst Reaction Examples Class 10.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Reaction Examples Class 10 Catalysts are substances which alter the rate of reaction by changing the path of reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance. Catalyst Reaction Examples Class 10.
From www.youtube.com
Chemical reaction and catalyst class 10th science YouTube Catalyst Reaction Examples Class 10 The reaction which involves a catalyst in their. A catalyst refers to a substance that can speed up a chemical reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are substances which alter the rate of reaction by changing the path of reaction.. Catalyst Reaction Examples Class 10.
From www.youtube.com
Chemical reaction and catalyst part 03 Competition Class 10th Catalyst Reaction Examples Class 10 A catalyst refers to a substance that can speed up a chemical reaction. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. The reaction which involves a catalyst in their. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are substances. Catalyst Reaction Examples Class 10.
From www.youtube.com
Chemical Reaction and equation (Exothermic and Endothermic Reaction Catalyst Reaction Examples Class 10 A catalyst refers to a substance that can speed up a chemical reaction. Learn what catalyst is in detail, types of catalyst along with important. Catalysts are substances which alter the rate of reaction by changing the path of reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations. Catalyst Reaction Examples Class 10.
From lessoncampusunspelt.z13.web.core.windows.net
Activity Of Enzyme Formula Catalyst Reaction Examples Class 10 Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The reaction which involves a catalyst in their. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are those substances which increase or decrease the rate of chemical. Catalyst Reaction Examples Class 10.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst Reaction Examples Class 10 This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Learn what catalyst is in detail, types of catalyst along with important. Catalysts are substances which alter the rate of reaction by changing the path of reaction. The reaction which involves a catalyst in their. Catalyst,. Catalyst Reaction Examples Class 10.
From www.mdpi.com
Catalysts Free FullText Recent Advances in Electrochemical Catalyst Reaction Examples Class 10 This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. A catalyst refers to a substance that can speed up a chemical reaction. Catalysts. Catalyst Reaction Examples Class 10.
From www.youtube.com
Natural and Synthetic Catalysts // Reaction Engineerin Class 144 Catalyst Reaction Examples Class 10 A catalyst refers to a substance that can speed up a chemical reaction. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are substances. Catalyst Reaction Examples Class 10.
From hxecfmpwa.blob.core.windows.net
Examples Of Catalysts In The Body at Christian Redmond blog Catalyst Reaction Examples Class 10 A catalyst refers to a substance that can speed up a chemical reaction. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. Learn what catalyst is in detail, types of catalyst along with important. Catalyst, in chemistry, any substance that increases the rate of a reaction without. Catalyst Reaction Examples Class 10.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Catalyst Reaction Examples Class 10 Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst refers to a substance that can speed up a chemical reaction. Catalysts are substances which alter the rate of reaction by changing. Catalyst Reaction Examples Class 10.
From msoid.ibuypower.com
Types Of Reactions And Balancing Worksheet Best Printable Resources Catalyst Reaction Examples Class 10 Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. Catalysts are substances which alter the rate of reaction by changing the path of reaction. The reaction which. Catalyst Reaction Examples Class 10.
From www.pinterest.com.mx
Catalysis Chemistry classroom, Chemistry Catalyst Reaction Examples Class 10 The reaction which involves a catalyst in their. Catalysts are substances which alter the rate of reaction by changing the path of reaction. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. A catalyst is a substance that is used to speed up a chemical reaction but it is. Catalyst Reaction Examples Class 10.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalyst Reaction Examples Class 10 Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst refers to a substance that can speed up a chemical reaction. Catalysts are substances which alter the rate of reaction by changing the path of reaction. The reaction which involves a catalyst in their. A catalyst is a substance that is used. Catalyst Reaction Examples Class 10.
From www.yumpu.com
CATALYTIC REFORMING Process,Catalysts and Reactors Catalyst Reaction Examples Class 10 This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. Catalysts are those substances which increase or decrease the rate of chemical reaction without. Catalyst Reaction Examples Class 10.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Reaction Examples Class 10 This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process.. Catalyst Reaction Examples Class 10.
From www.mdpi.com
Catalysts Free FullText General and Prospective Views on Oxidation Catalyst Reaction Examples Class 10 A catalyst refers to a substance that can speed up a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. Learn what catalyst is in detail, types of catalyst. Catalyst Reaction Examples Class 10.
From ar.inspiredpencil.com
Catalyst Examples For Kids Catalyst Reaction Examples Class 10 Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. Learn what catalyst is in detail, types of catalyst along with important. The reaction which involves a catalyst in their. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations. Catalyst Reaction Examples Class 10.
From www.youtube.com
Class 10th Chemical reaction and catalyst part 03 Double Catalyst Reaction Examples Class 10 A catalyst refers to a substance that can speed up a chemical reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are those substances which increase or decrease the rate of chemical reaction without getting consumed in the reaction process. Catalysts are substances. Catalyst Reaction Examples Class 10.