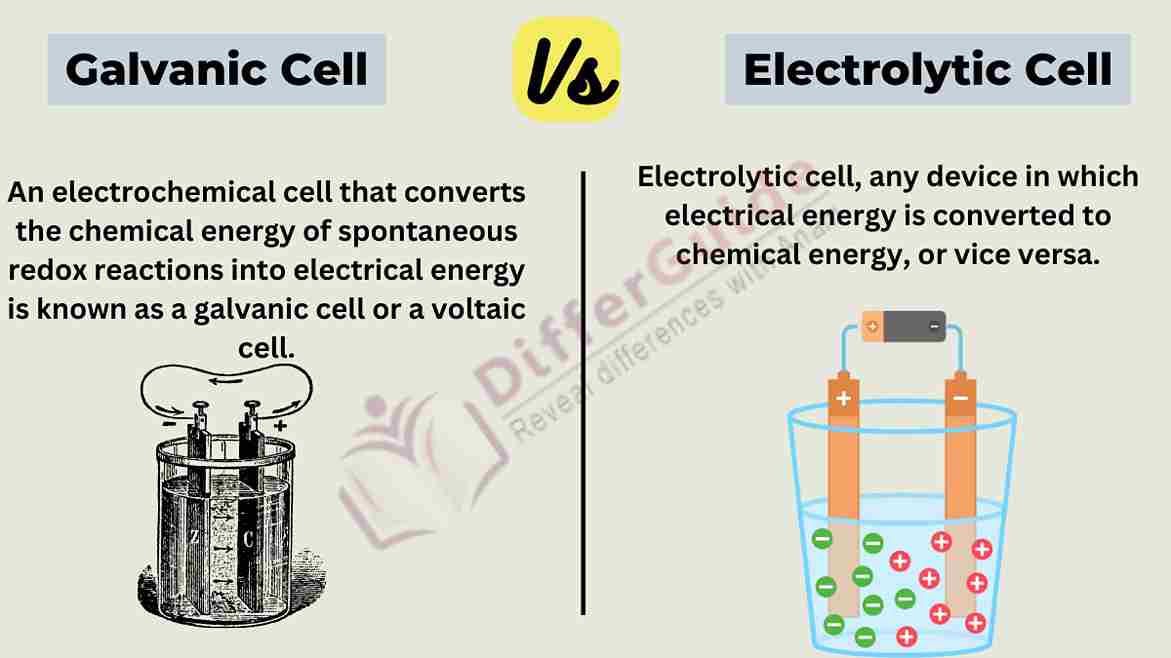
How Do Galvanic Cells Differ From Electrolytic Cells . The chemical energy is converted into. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. These are galvanic cells and electrolytic cells in detail. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In this article, we will study about two main cells; Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. Most galvanic cells contain two.
from differguide.com
A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. These are galvanic cells and electrolytic cells in detail. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In this article, we will study about two main cells; Most galvanic cells contain two. The chemical energy is converted into.
Difference Between Galvanic And Electrolytic Cell
How Do Galvanic Cells Differ From Electrolytic Cells Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In this article, we will study about two main cells; A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Most galvanic cells contain two. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The chemical energy is converted into. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. These are galvanic cells and electrolytic cells in detail.
From www.studypool.com
SOLUTION Electrochemical cells galvanic and electrolytic cell Studypool How Do Galvanic Cells Differ From Electrolytic Cells Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. In this article, we will study about two main cells; Most galvanic cells contain two.. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell How Do Galvanic Cells Differ From Electrolytic Cells These are galvanic cells and electrolytic cells in detail. The chemical energy is converted into. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. Most galvanic cells contain two. In this article, we will study about two main cells; A galvanic cell is an electrochemical cell in which spontaneous redox. How Do Galvanic Cells Differ From Electrolytic Cells.
From schoolbag.info
Electrochemistry Review the Knowledge You Need to Score High 5 How Do Galvanic Cells Differ From Electrolytic Cells Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In this article, we will study about two main cells; A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. These are galvanic cells and electrolytic cells in detail. In terms of differences, the most. How Do Galvanic Cells Differ From Electrolytic Cells.
From socratic.org
How can a galvanic cell an electrolytic cell? Socratic How Do Galvanic Cells Differ From Electrolytic Cells Most galvanic cells contain two. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. These are galvanic cells and electrolytic cells in detail. The chemical energy is converted into. In terms of differences,. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.chemistrylearner.com
Galvanic Cell (Voltaic Cell) Chemistry Learner How Do Galvanic Cells Differ From Electrolytic Cells These are galvanic cells and electrolytic cells in detail. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. In this article, we will study about two main cells; In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply. How Do Galvanic Cells Differ From Electrolytic Cells.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps How Do Galvanic Cells Differ From Electrolytic Cells The chemical energy is converted into. In this article, we will study about two main cells; In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of. How Do Galvanic Cells Differ From Electrolytic Cells.
From differguide.com
Difference Between Galvanic And Electrolytic Cell How Do Galvanic Cells Differ From Electrolytic Cells These are galvanic cells and electrolytic cells in detail. In this article, we will study about two main cells; Most galvanic cells contain two. The chemical energy is converted into. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. A galvanic cell. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.youtube.com
Galvanic Cell Vs Electrolytic Cell differences YouTube How Do Galvanic Cells Differ From Electrolytic Cells The chemical energy is converted into. These are galvanic cells and electrolytic cells in detail. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.differencebetween.net
Difference Between Galvanic Cells and Electrolytic Cells Difference How Do Galvanic Cells Differ From Electrolytic Cells Most galvanic cells contain two. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to. How Do Galvanic Cells Differ From Electrolytic Cells.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell How Do Galvanic Cells Differ From Electrolytic Cells In this article, we will study about two main cells; In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. The chemical energy is converted into. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow.. How Do Galvanic Cells Differ From Electrolytic Cells.
From jesse-has-lozano.blogspot.com
Explain the Difference Between a Galvanic Cell and Electrolytic Cell How Do Galvanic Cells Differ From Electrolytic Cells In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The chemical energy is converted into. These are galvanic cells and electrolytic cells in detail. Galvanic. How Do Galvanic Cells Differ From Electrolytic Cells.
From in.pinterest.com
Understanding Galvanic Cells A Simplified Guide How Do Galvanic Cells Differ From Electrolytic Cells Most galvanic cells contain two. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. Electrolytic cells and galvanic cells are both types of electrochemical. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation (Video) JoVE How Do Galvanic Cells Differ From Electrolytic Cells Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. These are galvanic cells and electrolytic cells in detail. Most galvanic cells contain two. The chemical energy is converted into. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. A galvanic cell. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle How Do Galvanic Cells Differ From Electrolytic Cells Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. These are galvanic cells and electrolytic cells in detail. In terms of differences, the most significant is that a galvanic cell utilizes a. How Do Galvanic Cells Differ From Electrolytic Cells.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell How Do Galvanic Cells Differ From Electrolytic Cells In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy. How Do Galvanic Cells Differ From Electrolytic Cells.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells How Do Galvanic Cells Differ From Electrolytic Cells These are galvanic cells and electrolytic cells in detail. In this article, we will study about two main cells; Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The chemical energy is converted. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.javatpoint.com
Difference Between Galvanic Cells and Electrolytic Cells javatpoint How Do Galvanic Cells Differ From Electrolytic Cells Most galvanic cells contain two. The chemical energy is converted into. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. These are galvanic. How Do Galvanic Cells Differ From Electrolytic Cells.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts How Do Galvanic Cells Differ From Electrolytic Cells In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. In this article, we will study about two main cells; Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. Most galvanic cells contain. How Do Galvanic Cells Differ From Electrolytic Cells.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell How Do Galvanic Cells Differ From Electrolytic Cells Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.goconqr.com
Electrochemistry Mind Map How Do Galvanic Cells Differ From Electrolytic Cells These are galvanic cells and electrolytic cells in detail. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The chemical energy is converted into. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In terms of differences, the most significant is that a. How Do Galvanic Cells Differ From Electrolytic Cells.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How How Do Galvanic Cells Differ From Electrolytic Cells The chemical energy is converted into. In this article, we will study about two main cells; Most galvanic cells contain two. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.youtube.com
Galvanic versus Electrolytic Cells YouTube How Do Galvanic Cells Differ From Electrolytic Cells In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. The chemical energy is converted into. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In this article, we will study about two main. How Do Galvanic Cells Differ From Electrolytic Cells.
From chem.libretexts.org
Electrolytic Cells Chemistry LibreTexts How Do Galvanic Cells Differ From Electrolytic Cells These are galvanic cells and electrolytic cells in detail. In this article, we will study about two main cells; Most galvanic cells contain two. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. In. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.slideserve.com
PPT Galvanic and Electrolytic Cells PowerPoint Presentation, free How Do Galvanic Cells Differ From Electrolytic Cells Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. These are galvanic cells and electrolytic cells in detail. The chemical energy is converted into. Most galvanic cells contain two. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.youtube.com
HOW GALVANIC CELL WORKS YouTube How Do Galvanic Cells Differ From Electrolytic Cells In this article, we will study about two main cells; The chemical energy is converted into. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with. How Do Galvanic Cells Differ From Electrolytic Cells.
From in.pinterest.com
three different types of electronic devices are shown in this diagram How Do Galvanic Cells Differ From Electrolytic Cells In this article, we will study about two main cells; Most galvanic cells contain two. These are galvanic cells and electrolytic cells in detail. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of electrical. The chemical energy is converted into. A galvanic cell is an electrochemical cell in which spontaneous redox processes. How Do Galvanic Cells Differ From Electrolytic Cells.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell How Do Galvanic Cells Differ From Electrolytic Cells In this article, we will study about two main cells; In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. Most galvanic cells contain two. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. How Do Galvanic Cells Differ From Electrolytic Cells.
From saylordotorg.github.io
Describing Electrochemical Cells How Do Galvanic Cells Differ From Electrolytic Cells The chemical energy is converted into. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. Most galvanic cells contain two. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download How Do Galvanic Cells Differ From Electrolytic Cells A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. The chemical energy is converted into. These are galvanic cells and electrolytic cells in detail. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. In terms of differences, the most significant is that. How Do Galvanic Cells Differ From Electrolytic Cells.
From allthedifferences.com
What Is The Difference Between Electrolytic Cells And Galvanic Cells How Do Galvanic Cells Differ From Electrolytic Cells These are galvanic cells and electrolytic cells in detail. In this article, we will study about two main cells; A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons. How Do Galvanic Cells Differ From Electrolytic Cells.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell How Do Galvanic Cells Differ From Electrolytic Cells The chemical energy is converted into. A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow. In this article, we will study about two main cells; These are galvanic cells and electrolytic cells in detail. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells How Do Galvanic Cells Differ From Electrolytic Cells Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. In terms of differences, the most significant is that a galvanic cell utilizes a spontaneous exothermic reaction to provide a steady supply of electrons with potential energy. The chemical energy is converted into. Most galvanic cells contain two. These are galvanic. How Do Galvanic Cells Differ From Electrolytic Cells.
From mungfali.com
Galvanic And Electrolytic Cells How Do Galvanic Cells Differ From Electrolytic Cells These are galvanic cells and electrolytic cells in detail. Most galvanic cells contain two. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. In this article, we will study about two main cells; A galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the continuous flow.. How Do Galvanic Cells Differ From Electrolytic Cells.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision How Do Galvanic Cells Differ From Electrolytic Cells Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. Most galvanic cells contain two. These are galvanic cells and electrolytic cells in detail. In this article, we will study about two main cells; Galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external source of. How Do Galvanic Cells Differ From Electrolytic Cells.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 How Do Galvanic Cells Differ From Electrolytic Cells Most galvanic cells contain two. The chemical energy is converted into. Electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical energy. In this article, we will study about two main cells; These are galvanic cells and electrolytic cells in detail. In terms of differences, the most significant is that a galvanic. How Do Galvanic Cells Differ From Electrolytic Cells.