Potentiometric Titration Equation . Instead, the electric potential across the substance is measured. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. From the resulting titration curves, you. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. It is used in the characterization of acids. In this method, there is no use of a chemical indicator. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Potentiometric titration is a laboratory method to determine the concentration of a given analyte.
from www.youtube.com
From the resulting titration curves, you. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Instead, the electric potential across the substance is measured. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. It is used in the characterization of acids. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. In this method, there is no use of a chemical indicator. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration.
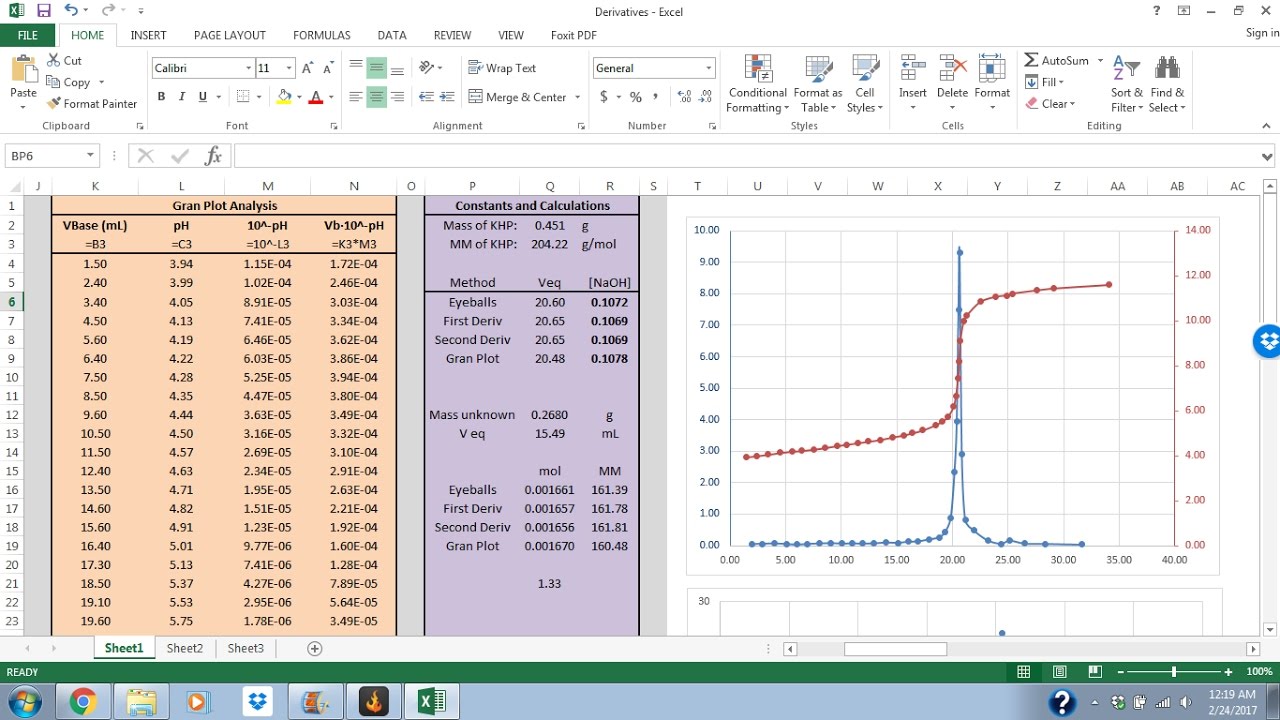
Excel Tutorial 2 Titration Analysis YouTube
Potentiometric Titration Equation Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. From the resulting titration curves, you. In this method, there is no use of a chemical indicator. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Instead, the electric potential across the substance is measured. It is used in the characterization of acids. Potentiometric titration is a laboratory method to determine the concentration of a given analyte.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Potentiometric Titration Equation Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. In this method, there is no use of a chemical indicator. It is used in the characterization of acids. Instead, the electric. Potentiometric Titration Equation.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Potentiometric Titration Equation It is used in the characterization of acids. From the resulting titration curves, you. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. In this method, there is no use of a chemical indicator. In. Potentiometric Titration Equation.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Potentiometric Titration Equation Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Potentiometric titration refers to a chemical method of. Potentiometric Titration Equation.
From mavink.com
Potentiometric Titration Curve Potentiometric Titration Equation In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. In this method, there is no use of a chemical indicator. From the resulting titration curves, you. Potentiometric titration can be used to determine the concentration of an. Potentiometric Titration Equation.
From www.researchgate.net
Examples of potentiometric titration curves of the used solvents to Potentiometric Titration Equation Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. From the resulting titration curves, you. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. In this method, there is no. Potentiometric Titration Equation.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Potentiometric Titration Equation Potentiometric titration is a laboratory method to determine the concentration of a given analyte. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. It is used in the characterization of acids. Instead, the electric potential across the substance is measured. Potentiometric titration can be used to determine the concentration of an. Potentiometric Titration Equation.
From pubs.acs.org
Determining a Solubility Product Constant by Potentiometric Titration Potentiometric Titration Equation In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. In this method, there is no use of a chemical indicator. Instead, the electric potential across the substance is measured. It is used in the characterization of acids. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect.. Potentiometric Titration Equation.
From studylib.net
2 POTENTIOMETRIC TITRATIONS Potentiometric Titration Equation It is used in the characterization of acids. Instead, the electric potential across the substance is measured. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration is a laboratory. Potentiometric Titration Equation.
From www.researchgate.net
Potentiometric titrations of 1.0 mmol·dm −3 [Pt(SMC)(H 2 O) 2 ] + with Potentiometric Titration Equation In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. From the resulting titration curves, you. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. It is used in the characterization of acids.. Potentiometric Titration Equation.
From www.tessshebaylo.com
Karl Fischer Titration Equation Tessshebaylo Potentiometric Titration Equation Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Instead, the electric potential across the substance is measured. It is used in the characterization of acids. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric. Potentiometric Titration Equation.
From en.ppt-online.org
Electroanalytical Chemistry online presentation Potentiometric Titration Equation It is used in the characterization of acids. In this method, there is no use of a chemical indicator. From the resulting titration curves, you. Instead, the electric potential across the substance is measured. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration can be used to determine the concentration of an electrolytic. Potentiometric Titration Equation.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID737892 Potentiometric Titration Equation Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. From the resulting titration curves, you. It is used in the characterization of acids. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Instead, the electric potential across the substance is. Potentiometric Titration Equation.
From mavink.com
Titration Labeled Potentiometric Titration Equation It is used in the characterization of acids. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. From the resulting titration curves, you. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using. Potentiometric Titration Equation.
From www.studypool.com
SOLUTION Experiment of potentiometric titration Studypool Potentiometric Titration Equation It is used in the characterization of acids. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration involves the measurement of the potential of an. Potentiometric Titration Equation.
From www.scielo.br
SciELO Brasil Potentiometric Titration and OutOfEquilibrium pH Potentiometric Titration Equation Instead, the electric potential across the substance is measured. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. It is used in the characterization of acids. In this experiment, we will use the electrochemical cell. Potentiometric Titration Equation.
From www.youtube.com
Potentiometric Titration of Iron YouTube Potentiometric Titration Equation From the resulting titration curves, you. In this method, there is no use of a chemical indicator. Instead, the electric potential across the substance is measured. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Potentiometric titration. Potentiometric Titration Equation.
From www.researchgate.net
Typical curves of potentiometric titrations starting from the PZC Potentiometric Titration Equation Instead, the electric potential across the substance is measured. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. In this method, there is no use of a chemical indicator. Potentiometric titration refers to a chemical method of. Potentiometric Titration Equation.
From www.youtube.com
Chem L7 Part 2 Potentiometric Titration YouTube Potentiometric Titration Equation In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. In this method, there is no use of a chemical indicator. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. It is used. Potentiometric Titration Equation.
From www.scribd.com
POTENTIOMETRY PDF Titration Chemistry Potentiometric Titration Equation From the resulting titration curves, you. It is used in the characterization of acids. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Instead, the electric potential across the substance is measured. Potentiometric titration involves the measurement of the potential of an indicator electrode with. Potentiometric Titration Equation.
From studylib.net
4. Potentiometry Potentiometric Titration Equation From the resulting titration curves, you. In this method, there is no use of a chemical indicator. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an. Potentiometric Titration Equation.
From www.youtube.com
Potentiometric acid base titrations YouTube Potentiometric Titration Equation Potentiometric titration is a laboratory method to determine the concentration of a given analyte. In this method, there is no use of a chemical indicator. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. From the resulting titration curves, you. Instead, the electric potential across the substance is measured.. Potentiometric Titration Equation.
From www.researchgate.net
Potentiometric titration curves and first derivates of native and Potentiometric Titration Equation It is used in the characterization of acids. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is. Potentiometric Titration Equation.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Potentiometric Titration Equation In this method, there is no use of a chemical indicator. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration is a laboratory method to determine the concentration of. Potentiometric Titration Equation.
From www.youtube.com
Potentiometric titration procedure YouTube Potentiometric Titration Equation In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Instead, the electric potential across the substance is measured. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Potentiometric titration refers to a chemical method of analysis. Potentiometric Titration Equation.
From www.slideserve.com
PPT POTENTIOMETRY 9 th lecture PowerPoint Presentation, free download Potentiometric Titration Equation Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. From the resulting titration curves, you. In this method, there is no use of a chemical. Potentiometric Titration Equation.
From ceias.nau.edu
Potentiometry Potentiometric Titration Equation Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. From the resulting titration curves, you. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Instead, the electric potential across the substance is measured. In this experiment, we will use the. Potentiometric Titration Equation.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Potentiometric Titration Equation In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. From the resulting titration curves, you. It is used in the characterization of acids. In this method, there is no use of a chemical indicator. Potentiometric titration refers. Potentiometric Titration Equation.
From www.studocu.com
Nernst Equation ApplicationsPotentiometric titrations APPLICATIONS Potentiometric Titration Equation In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Instead, the electric potential. Potentiometric Titration Equation.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Potentiometric Titration Equation In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. From the resulting titration curves, you. Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration refers to a chemical method of. Potentiometric Titration Equation.
From www.studocu.com
Experiment 4 Estimation of Fe(II) in a solution using potentiometric Potentiometric Titration Equation Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. In this method, there is no use of a chemical indicator. Potentiometric titration is a laboratory. Potentiometric Titration Equation.
From www.vernier.com
Potentiometric Titration of Aqueous Iodine Potentiometric Titration Equation Potentiometric titration is a laboratory method to determine the concentration of a given analyte. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Instead, the electric potential. Potentiometric Titration Equation.
From www.youtube.com
Excel Tutorial 2 Titration Analysis YouTube Potentiometric Titration Equation In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. In this method, there is no use of a chemical indicator. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Instead, the electric potential across the substance. Potentiometric Titration Equation.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 Potentiometric Titration Equation Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Instead, the electric potential across the substance is measured. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. It is used. Potentiometric Titration Equation.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Potentiometric Titration Equation Potentiometric titration is a laboratory method to determine the concentration of a given analyte. From the resulting titration curves, you. Instead, the electric potential across the substance is measured. In this experiment, we will use the electrochemical cell as a method of chemical analysis called potentiometric titration. In this method, there is no use of a chemical indicator. Potentiometric titration. Potentiometric Titration Equation.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID4072119 Potentiometric Titration Equation Potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. It is. Potentiometric Titration Equation.