J&J Prostate Cancer Drug . The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer.
from oncpracticemanagement.com
The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit.
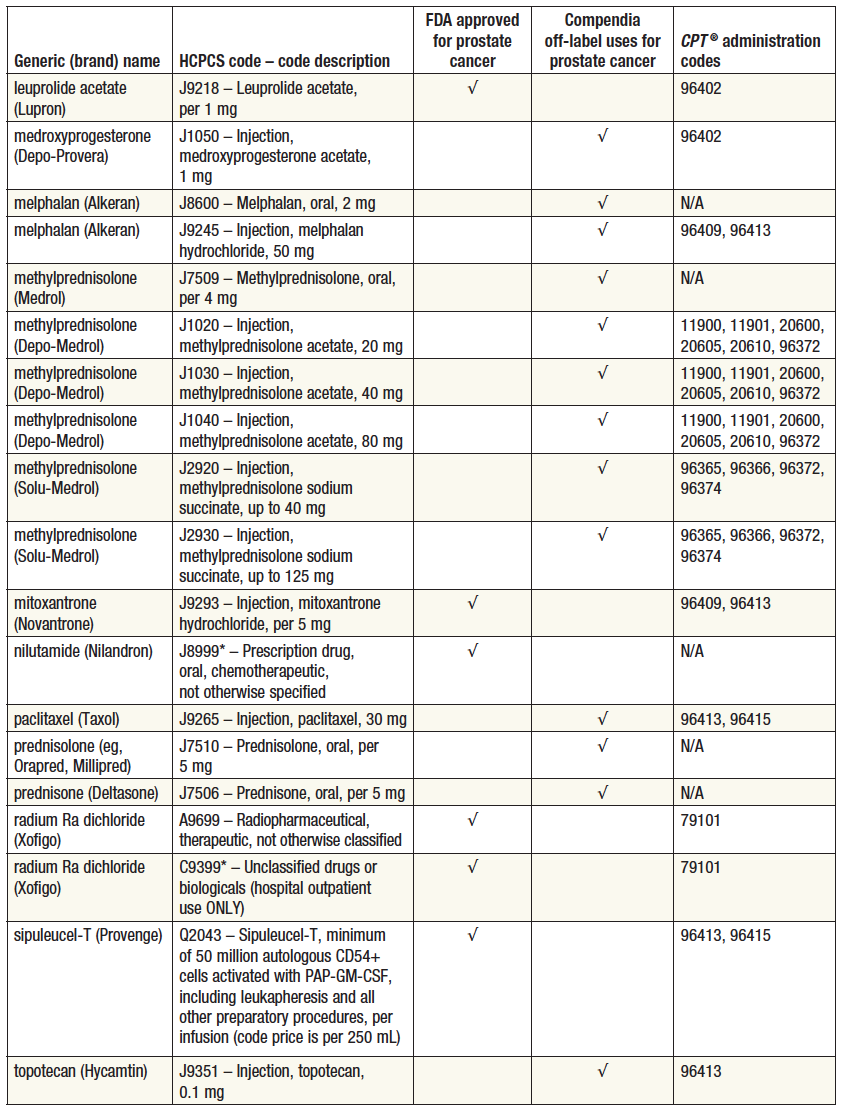
FDAApproved Medications Used for the Treatment of Prostate Cancer
J&J Prostate Cancer Drug Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application.
From www.deccanherald.com
BDR Pharma launches 80 mg version of prostate cancer drug at Rs 24,480 J&J Prostate Cancer Drug The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Janssen, a subsidiary of johnson & johnson jnj, announced that. J&J Prostate Cancer Drug.
From actchealth.com
Prostate Cancer & Treatment A Comprehensive Guide ACTC J&J Prostate Cancer Drug Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Final analysis from phase 3 titan study demonstrated erleada® provided. J&J Prostate Cancer Drug.
From cancerdigest.blogspot.com
Cancer Digest Prostate Cancer J&J Prostate Cancer Drug Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has. J&J Prostate Cancer Drug.
From www.pharmacompass.com
FDA grants accelerated nods to J&J, Pfizer’s blood cancer therapies J&J Prostate Cancer Drug The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Final analysis from phase. J&J Prostate Cancer Drug.
From nai500.com
FDA approves J&J prostate cancer treatment NAI 500 J&J Prostate Cancer Drug Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate. J&J Prostate Cancer Drug.
From www.withpower.com
Treatment (chemotherapy and enzyme inhibitor) for Prostate Cancer J&J Prostate Cancer Drug Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Certain gene mutations can dramatically increase the. J&J Prostate Cancer Drug.
From www.newsmax.com
New Drugs May Offer Prostate Cancer Cure J&J Prostate Cancer Drug Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone. J&J Prostate Cancer Drug.
From www.behance.net
J&J Prostate Cancer on Behance J&J Prostate Cancer Drug Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has. J&J Prostate Cancer Drug.
From www.oncpracticemanagement.com
FDAApproved Medications Used for the Treatment of Prostate Cancer J&J Prostate Cancer Drug Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. The fda on friday signed off on janssen. J&J Prostate Cancer Drug.
From www.medindia.net
Drugs for Prostate Cancer Treatment, Side Effects & Precautions J&J Prostate Cancer Drug Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Certain gene mutations can. J&J Prostate Cancer Drug.
From www.drugdiscoverytrends.com
J&J's ERLEADA Lowered Risk of Death in Patients with NonMetastatic J&J Prostate Cancer Drug Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. The fda on friday signed off on janssen. J&J Prostate Cancer Drug.
From nytimes.com
Zytiga, a Prostate Cancer Drug, Does Well in Trial The New York Times J&J Prostate Cancer Drug Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Certain gene mutations can dramatically increase the. J&J Prostate Cancer Drug.
From oncpracticemanagement.com
FDAApproved Medications Used for the Treatment of Prostate Cancer J&J Prostate Cancer Drug Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Certain gene mutations can. J&J Prostate Cancer Drug.
From www.uspharmacist.com
An Overview of Prostate Cancer J&J Prostate Cancer Drug Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Final analysis from phase. J&J Prostate Cancer Drug.
From www.publichealthnotes.com
Prostate Cancer Causes, Types and Treatment Public Health Notes J&J Prostate Cancer Drug Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate. J&J Prostate Cancer Drug.
From www.quantib.com
How can urologists improve patient care in the prostate cancer pathway? J&J Prostate Cancer Drug Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has. J&J Prostate Cancer Drug.
From www.slideserve.com
PPT New Drug for Prostate Cancer Gets FDA Nod PowerPoint Presentation J&J Prostate Cancer Drug The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Certain gene mutations can. J&J Prostate Cancer Drug.
From dailynews.ascopubs.org
Risks and Benefits of Immunotherapy Versus Chemotherapy in Patients J&J Prostate Cancer Drug Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Final analysis from phase 3 titan study demonstrated erleada®. J&J Prostate Cancer Drug.
From english.radio.cz
European Commission approves revolutionary prostate cancer drug co J&J Prostate Cancer Drug Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Johnson & johnson ’s jnj subsidiary, janssen biotech announced. J&J Prostate Cancer Drug.
From www.pharmiweb.com
Prostate Cancer Drug Development Summit J&J Prostate Cancer Drug The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Johnson & johnson ’s jnj subsidiary, janssen. J&J Prostate Cancer Drug.
From www.youtube.com
Posluma A Breakthrough Drug for Prostate Cancer who fda medicine J&J Prostate Cancer Drug Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Final analysis from phase 3 titan study demonstrated erleada® provided. J&J Prostate Cancer Drug.
From www.mdpi.com
IJMS Free FullText Treating Prostate Cancer by AntibodyDrug J&J Prostate Cancer Drug Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted. J&J Prostate Cancer Drug.
From www.researchandmarkets.com
Metastatic Prostate Cancer Drugs in Development by Stages, Target, MoA J&J Prostate Cancer Drug Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Final analysis from phase 3 titan study demonstrated erleada®. J&J Prostate Cancer Drug.
From synapse.zhihuiya.com
Janssen launches oncedaily option for prostate cancer drug Erleada J&J Prostate Cancer Drug Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Johnson & johnson ’s jnj subsidiary, janssen biotech announced. J&J Prostate Cancer Drug.
From www.researchgate.net
An Overview of Approved Drugs for Each State of Prostate Cancer Disease J&J Prostate Cancer Drug The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Janssen, a subsidiary of johnson & johnson jnj, announced that. J&J Prostate Cancer Drug.
From www.pharmatutor.org
Breakthrough discovery to transform prostate cancer treatment PharmaTutor J&J Prostate Cancer Drug Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has. J&J Prostate Cancer Drug.
From medizzy.com
List of the chemotherapy drugs for prostate cancer MEDizzy J&J Prostate Cancer Drug Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Certain gene mutations can dramatically increase the. J&J Prostate Cancer Drug.
From www.crownbio.cn
parpinhibitorsinprostatecancertreatment Crown Bioscience J&J Prostate Cancer Drug The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has. J&J Prostate Cancer Drug.
From oncpracticemanagement.com
FDAApproved Medications Used for the Treatment of Prostate Cancer J&J Prostate Cancer Drug Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. The fda on friday signed off on janssen. J&J Prostate Cancer Drug.
From www.permanenthire.co.uk
NHS fast tracks lifeextending prostate cancer drug to patients J&J Prostate Cancer Drug Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. The fda on friday signed off on janssen pharmaceutical ’s. J&J Prostate Cancer Drug.
From www.researchgate.net
Selected clinical studies focusing on prostate cancer with drugs J&J Prostate Cancer Drug Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Johnson & johnson ’s jnj subsidiary, janssen biotech announced. J&J Prostate Cancer Drug.
From health.economictimes.indiatimes.com
Bdr Pharmaceuticals BDR Pharma introduces prostate cancer drug with J&J Prostate Cancer Drug The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Janssen, a subsidiary of johnson & johnson jnj,. J&J Prostate Cancer Drug.
From oncpracticemanagement.com
FDAApproved Medications Used for the Treatment of Prostate Cancer J&J Prostate Cancer Drug The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has approved its new drug application. Final analysis from phase 3 titan study demonstrated erleada® provided. J&J Prostate Cancer Drug.
From www.independent.co.uk
Prostate cancer The two drugs that can radically delay the spread of J&J Prostate Cancer Drug The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. Janssen, a subsidiary of johnson & johnson jnj, announced that the fda has granted breakthrough therapy designation for parp. Johnson & johnson ’s jnj subsidiary, janssen biotech announced. J&J Prostate Cancer Drug.
From www.prostate-treatment-options.com
Prostate drugs Your mini guide J&J Prostate Cancer Drug Final analysis from phase 3 titan study demonstrated erleada® provided statistically significant overall survival benefit. Certain gene mutations can dramatically increase the risk of developing aggressive prostate cancer. The fda on friday signed off on janssen pharmaceutical ’s niraparib and abiraterone acetate tablets, now to be marketed. Johnson & johnson ’s jnj subsidiary, janssen biotech announced that the fda has. J&J Prostate Cancer Drug.