How To Calculate Acid Concentration From Titration . In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. \ce{naoh}\) is required to reach the end. by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. \ce{naoh}\) is required to reach the end. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. suppose that a titration is performed and \(20.70 \: suppose that a titration is performed and \(20.70 \:
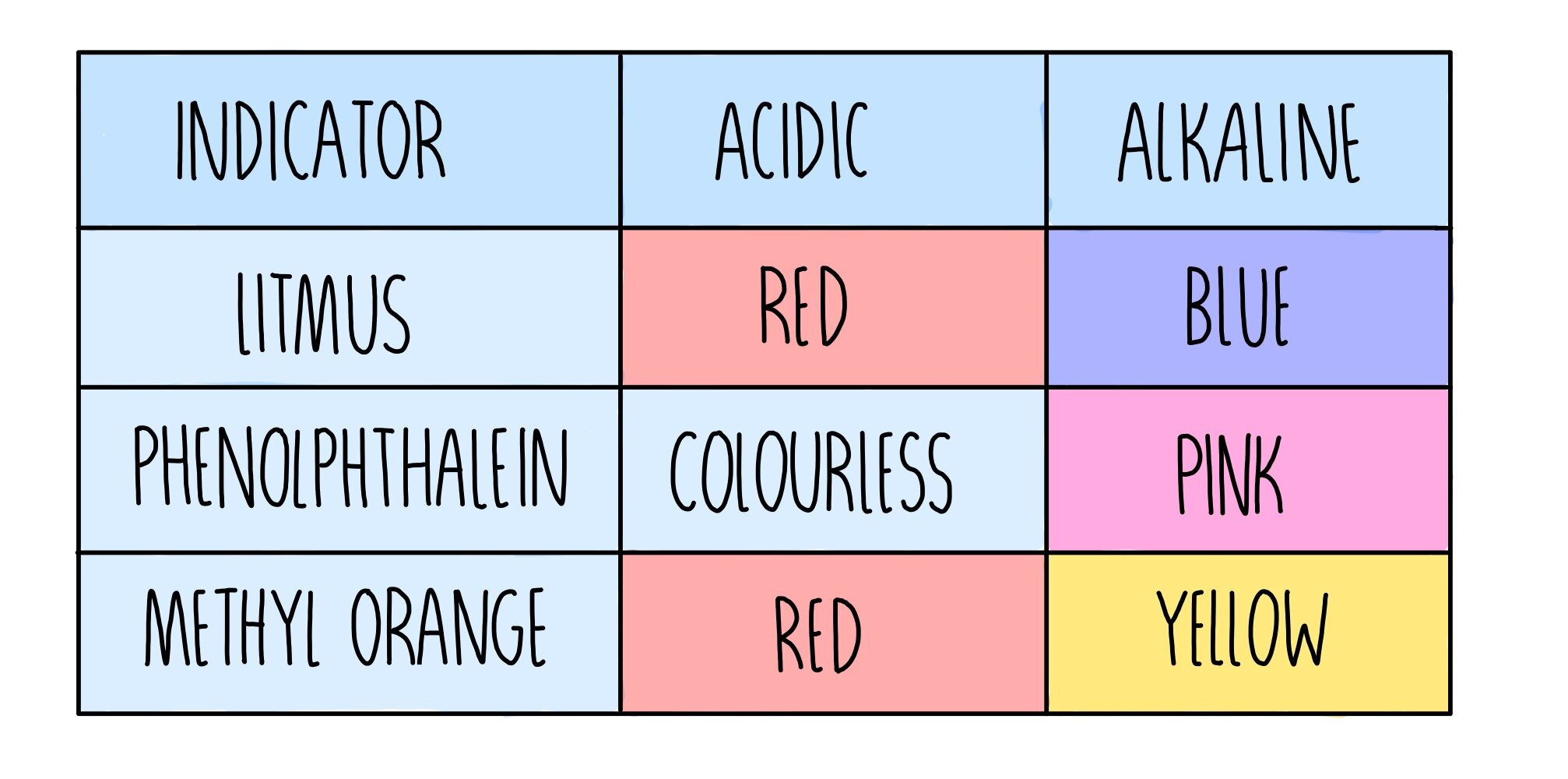
from www.thesciencehive.co.uk
suppose that a titration is performed and \(20.70 \: suppose that a titration is performed and \(20.70 \: calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. \ce{naoh}\) is required to reach the end. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. \ce{naoh}\) is required to reach the end.
Acids, Alkalis and Titrations* — the science sauce
How To Calculate Acid Concentration From Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. suppose that a titration is performed and \(20.70 \: suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. \ce{naoh}\) is required to reach the end.
From giorvlvoc.blob.core.windows.net
Acid Base Titration Curve at Adrian Yount blog How To Calculate Acid Concentration From Titration calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. suppose that a titration is performed and \(20.70 \: by knowing the volumes of acid and base. How To Calculate Acid Concentration From Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate Acid Concentration From Titration \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: suppose that a titration is performed and \(20.70 \: by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide. How To Calculate Acid Concentration From Titration.
From chemistry.stackexchange.com
equilibrium How to calculate the dissociation constant of a weak acid How To Calculate Acid Concentration From Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. suppose that a titration is performed and \(20.70 \: suppose that a titration is performed and \(20.70 \: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. \ce{naoh}\) is required to reach the end. calculate the ph. How To Calculate Acid Concentration From Titration.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. by knowing the volumes of acid and base. How To Calculate Acid Concentration From Titration.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations How To Calculate Acid Concentration From Titration \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. M_av_a = m_bv_b let's assume you are titrating a strong acid (10. How To Calculate Acid Concentration From Titration.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. \ce{naoh}\) is required to reach the end. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. \ce{naoh}\). How To Calculate Acid Concentration From Titration.
From www.slideshare.net
How do we find unknown concentrations of acids How To Calculate Acid Concentration From Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. \ce{naoh}\) is required to reach the end. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. suppose that. How To Calculate Acid Concentration From Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How To Calculate Acid Concentration From Titration \ce{naoh}\) is required to reach the end. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration.. How To Calculate Acid Concentration From Titration.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. \ce{naoh}\) is required to reach the end. \ce{naoh}\) is required to reach the end. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. How To Calculate Acid Concentration From Titration.
From quizpseudology.z21.web.core.windows.net
How To Find Equivalence Point Of A Weak Acid How To Calculate Acid Concentration From Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. \ce{naoh}\) is required to reach the end. by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end.. How To Calculate Acid Concentration From Titration.
From brainly.com
The graphs labeled (a) and (b) show the titration curves for two equal How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. In a. How To Calculate Acid Concentration From Titration.
From dxonsvczs.blob.core.windows.net
Titration Base Concentration at James Cummings blog How To Calculate Acid Concentration From Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. \ce{naoh}\) is required to reach the end. by knowing the volumes of acid and base used, and the concentration of. How To Calculate Acid Concentration From Titration.
From www.numerade.com
SOLVED The flask contains 25 mL of an unknown diprotic acid aqueous How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: suppose that a titration is performed and \(20.70 \: by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. \ce{naoh}\) is required to reach the end. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide. How To Calculate Acid Concentration From Titration.
From fr.wikihow.com
5 manières de calculer la concentration d'une solution How To Calculate Acid Concentration From Titration calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. \ce{naoh}\) is required to reach the end. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. \ce{naoh}\) is required to reach the end. suppose that a. How To Calculate Acid Concentration From Titration.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations* — the science sauce How To Calculate Acid Concentration From Titration \ce{naoh}\) is required to reach the end. by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. calculate the ph for the weak acid/strong. How To Calculate Acid Concentration From Titration.
From www.compoundchem.com
A Guide to Acids, Acid Strength, and Concentration Compound Interest How To Calculate Acid Concentration From Titration by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. suppose that a titration is performed and \(20.70 \: calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. \ce{naoh}\) is. How To Calculate Acid Concentration From Titration.
From freeonlinehelpns.blogspot.com
Free Online Help QThe titration of 10.00 mL of a diprotic acid How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. \ce{naoh}\) is required to reach the end. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. suppose that a titration is performed and \(20.70 \: M_av_a = m_bv_b let's assume you are titrating a strong acid (10. How To Calculate Acid Concentration From Titration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Calculate Acid Concentration From Titration calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. suppose that a titration is performed and \(20.70 \: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. In a titration, 25.0 cm 3 of 0.100. How To Calculate Acid Concentration From Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide. How To Calculate Acid Concentration From Titration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax How To Calculate Acid Concentration From Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. \ce{naoh}\). How To Calculate Acid Concentration From Titration.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog How To Calculate Acid Concentration From Titration by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. suppose that a titration is performed and \(20.70 \: suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach. How To Calculate Acid Concentration From Titration.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. \ce{naoh}\) is required to reach the end. M_av_a = m_bv_b let's assume you are titrating. How To Calculate Acid Concentration From Titration.
From mungfali.com
Acid Base Titration Formula How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. by knowing the volumes of acid and base used, and the. How To Calculate Acid Concentration From Titration.
From chemistry.stackexchange.com
Why does the pH before the equivalence point of a titration depend on How To Calculate Acid Concentration From Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. . How To Calculate Acid Concentration From Titration.
From giorvlvoc.blob.core.windows.net
Acid Base Titration Curve at Adrian Yount blog How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. In a titration, 25.0 cm 3 of 0.100. How To Calculate Acid Concentration From Titration.
From ar.inspiredpencil.com
Titration Curve Acetic Acid How To Calculate Acid Concentration From Titration \ce{naoh}\) is required to reach the end. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. suppose that a titration is performed and \(20.70 \: \ce{naoh}\). How To Calculate Acid Concentration From Titration.
From www.youtube.com
Finding concentration of acid or base given amount needed to titrate How To Calculate Acid Concentration From Titration \ce{naoh}\) is required to reach the end. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: calculate the ph for the weak acid/strong base titration between 50.0. How To Calculate Acid Concentration From Titration.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic How To Calculate Acid Concentration From Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations. How To Calculate Acid Concentration From Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during How To Calculate Acid Concentration From Titration calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. by. How To Calculate Acid Concentration From Titration.
From www.hotzxgirl.com
Solved Titration Standardization Of Sodium Hydroxide Submit Chegg Hot How To Calculate Acid Concentration From Titration \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. In. How To Calculate Acid Concentration From Titration.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How To Calculate Acid Concentration From Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. M_av_a = m_bv_b let's assume you are titrating. How To Calculate Acid Concentration From Titration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation How To Calculate Acid Concentration From Titration suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. \ce{naoh}\) is required to reach the end. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. by. How To Calculate Acid Concentration From Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts How To Calculate Acid Concentration From Titration by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. suppose that a titration is performed and \(20.70 \: suppose. How To Calculate Acid Concentration From Titration.
From mungfali.com
Acid Base Titration Calculation How To Calculate Acid Concentration From Titration \ce{naoh}\) is required to reach the end. calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the listed. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. M_av_a = m_bv_b let's assume you are titrating a strong acid (10. How To Calculate Acid Concentration From Titration.
From learningschoolmurgkerny1.z13.web.core.windows.net
Titration Practical Questions And Answers Pdf How To Calculate Acid Concentration From Titration M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. \ce{naoh}\) is required to reach the end. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: by knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to.. How To Calculate Acid Concentration From Titration.