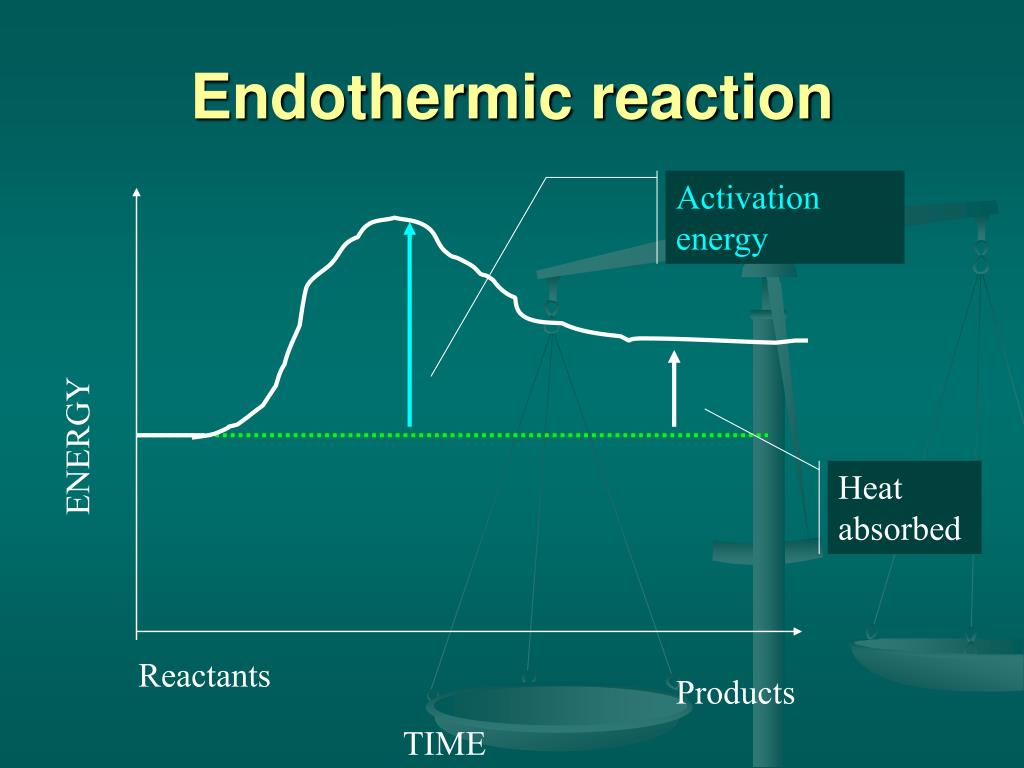
Endothermic Reaction Also Known As . An endothermic process does not involve a. E n d o t h e r m i c r e a c t i o n. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Reactants + energy (heat) → products.
from www.slideserve.com
an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. An endothermic process does not involve a. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Reactants + energy (heat) → products. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. E n d o t h e r m i c r e a c t i o n.
PPT Chemical Reactions PowerPoint Presentation, free download ID431456
Endothermic Reaction Also Known As in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Reactants + energy (heat) → products. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. An endothermic process does not involve a. E n d o t h e r m i c r e a c t i o n.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Also Known As E n d o t h e r m i c r e a c t i o n. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to. Endothermic Reaction Also Known As.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction Also Known As E n d o t h e r m i c r e a c t i o n. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Reactants + energy (heat) → products. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. . Endothermic Reaction Also Known As.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Also Known As Reactants + energy (heat) → products. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. an endothermic reaction is a. Endothermic Reaction Also Known As.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID5403517 Endothermic Reaction Also Known As in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. An endothermic process does not involve a. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. endothermic reactions are chemical reactions in. Endothermic Reaction Also Known As.
From facts.net
16 Intriguing Facts About Endothermic Endothermic Reaction Also Known As an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. revise and understand what endothermic and exothermic reactions are and how the two. Endothermic Reaction Also Known As.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation Endothermic Reaction Also Known As Reactants + energy (heat) → products. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. revise and understand what endothermic and exothermic reactions. Endothermic Reaction Also Known As.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Reaction Also Known As An endothermic process does not involve a. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.. Endothermic Reaction Also Known As.
From techschematic.com
The Enthalpy Diagram of an Endothermic Reaction Explained Endothermic Reaction Also Known As Reactants + energy (heat) → products. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. E n d o t h e r m i c r e a c t i o n. in endothermic reactions, more energy is absorbed when the bonds in the. Endothermic Reaction Also Known As.
From slideplayer.com
THERMOCHEMISTRY Thermodynamics ppt download Endothermic Reaction Also Known As endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. E n d o t h e r m i c r. Endothermic Reaction Also Known As.
From avopix.com
Exothermic VS Endothermic Reaction Vector Royalty Free Stock Vector Endothermic Reaction Also Known As revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Reactants + energy (heat) → products. An endothermic process does not involve a. endothermic reactions are chemical reactions in which the reactants absorb heat energy. Endothermic Reaction Also Known As.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Endothermic Reaction Also Known As E n d o t h e r m i c r e a c t i o n. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Reactants + energy (heat) → products. endothermic reactions are chemical reactions in which the reactants absorb heat energy. Endothermic Reaction Also Known As.
From slideplayer.com
Chemistry Notes Chapter 2 ppt download Endothermic Reaction Also Known As an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. An endothermic process does not involve a. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction. Endothermic Reaction Also Known As.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Also Known As in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. an endothermic reaction is a chemical change in which the system absorbs thermal. Endothermic Reaction Also Known As.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID431456 Endothermic Reaction Also Known As These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. An endothermic process does not involve a. E n d o t h e r m i c r e a c t i o n.. Endothermic Reaction Also Known As.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Also Known As in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. endothermic and exothermic reactions can be thought of as having energy as either. Endothermic Reaction Also Known As.
From www.slideserve.com
PPT Endothermic and Exothermic reactions PowerPoint Presentation Endothermic Reaction Also Known As in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. E n d o t h e r m i c r e a c t i o n. An endothermic process does not involve a. These reactions lower the temperature of their surrounding. Endothermic Reaction Also Known As.
From slidetodoc.com
Endothermic Exothermic Reactions Enthalpy H Heat Enthalpy Change Endothermic Reaction Also Known As An endothermic process does not involve a. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. E n d o t h e r m i c r e a c t i o n. in endothermic reactions, more energy is absorbed when the bonds in. Endothermic Reaction Also Known As.
From slideplayer.com
THERMOCHEMISTRY THERMODYNAMICS. ppt download Endothermic Reaction Also Known As endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. E n d o t h e r m i c r e a c t i o n. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product.. Endothermic Reaction Also Known As.
From www.slideserve.com
PPT 5.1 Exothermic and endothermic reactions PowerPoint Presentation Endothermic Reaction Also Known As revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. E n d o t h e r m i c r e a c t i o n. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a. Endothermic Reaction Also Known As.
From slideplayer.com
Endothermic and exothermic reactions ppt download Endothermic Reaction Also Known As An endothermic process does not involve a. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. E n d o t h e r m i c r e a c t i o n.. Endothermic Reaction Also Known As.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Also Known As These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. An endothermic process does not involve a. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. E n d o t h e r m i c r e a c t i o n.. Endothermic Reaction Also Known As.
From exymvnjmo.blob.core.windows.net
Endothermic Reaction Examples at Grace Mai blog Endothermic Reaction Also Known As E n d o t h e r m i c r e a c t i o n. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. . Endothermic Reaction Also Known As.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Also Known As These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Reactants + energy (heat) → products. . Endothermic Reaction Also Known As.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Also Known As endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. An endothermic process does not involve a. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Reactants + energy (heat) → products. in endothermic reactions, more. Endothermic Reaction Also Known As.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Reaction Also Known As revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Reactants + energy (heat) → products. in endothermic reactions, more energy is absorbed when the bonds in. Endothermic Reaction Also Known As.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Also Known As endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. An endothermic process does not involve a. E n d o t h e r m i c r e a c t i. Endothermic Reaction Also Known As.
From exyplunql.blob.core.windows.net
Endothermic Reaction Equilibrium Shift at Stewart Phillips blog Endothermic Reaction Also Known As Reactants + energy (heat) → products. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form. Endothermic Reaction Also Known As.
From www.facebook.com
What Are Endothermic & Exothermic Reactions Reactions Chemistry Endothermic Reaction Also Known As Reactants + energy (heat) → products. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. in endothermic reactions, more energy is absorbed when the bonds in the. Endothermic Reaction Also Known As.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Also Known As Reactants + energy (heat) → products. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. E n d o t h e r m i c r e a c t i o n. These reactions lower the temperature of their surrounding area, thereby creating a cooling. Endothermic Reaction Also Known As.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Endothermic Reaction Also Known As endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. An endothermic process does not involve a. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or.. Endothermic Reaction Also Known As.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Also Known As revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in endothermic reactions, more energy is. Endothermic Reaction Also Known As.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Also Known As in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. An endothermic process does not involve a. endothermic and exothermic reactions can be thought of as having energy as. Endothermic Reaction Also Known As.
From slidetodoc.com
Exothermic and Endothermic reactions Learning Objectives Explain heat Endothermic Reaction Also Known As in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. E n d o t h e r m i c r e a c t i o n. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the. Endothermic Reaction Also Known As.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation Endothermic Reaction Also Known As an endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in. endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form. Endothermic Reaction Also Known As.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Endothermic Reaction Also Known As endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the. These reactions lower the temperature of their surrounding area, thereby creating a. Endothermic Reaction Also Known As.