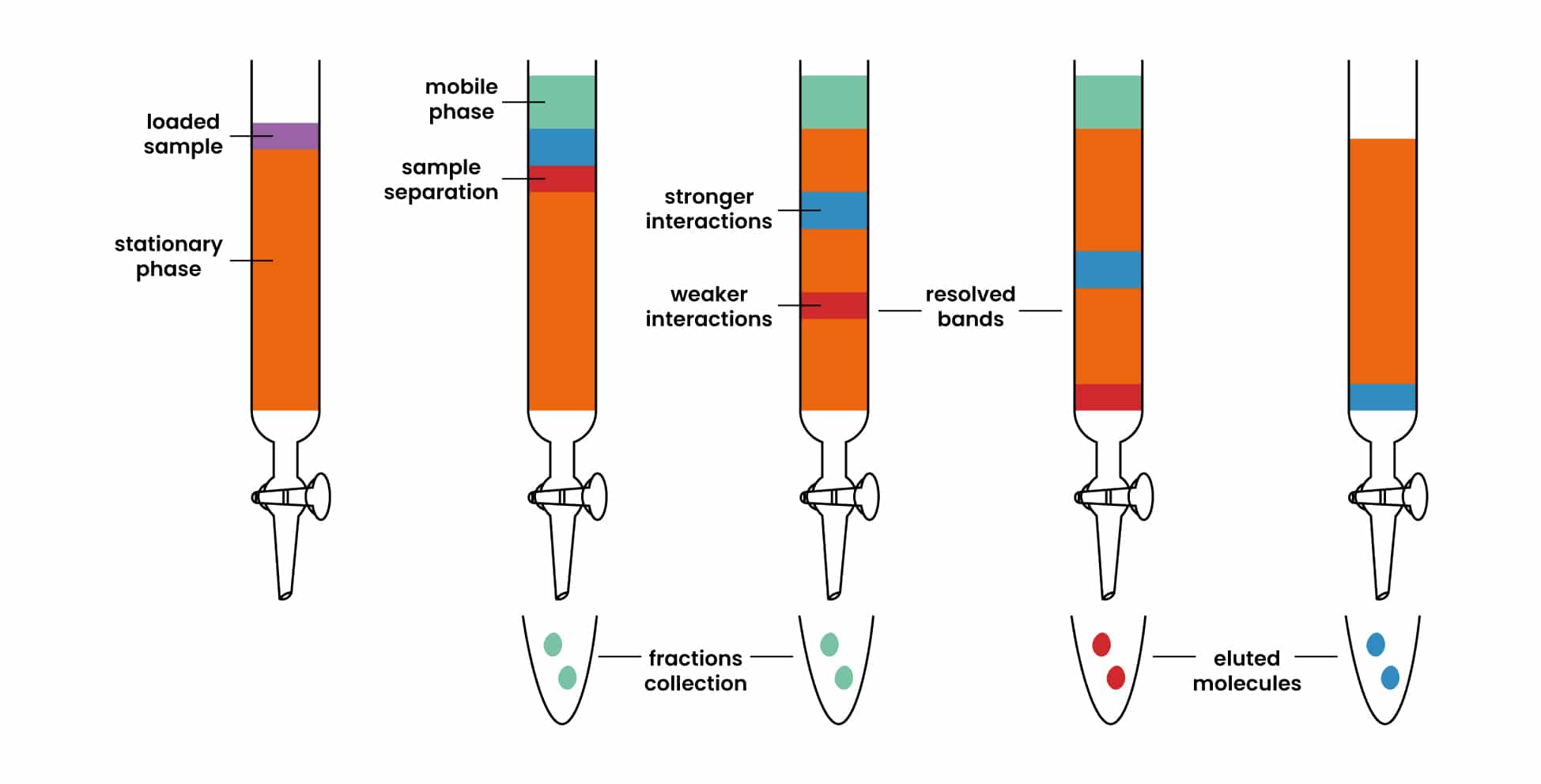
Filtration Vs Distillation And Chromatography . while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. Filtration separates solids of different sizes. Distillation takes advantage of differences in boiling points. Distillation (as discussed in analysis:. Evaporation removes a liquid from a solution to leave a solid material. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. chromatography involves solvent separation on a solid medium.
from www.slidemake.com
there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Filtration separates solids of different sizes. Evaporation removes a liquid from a solution to leave a solid material. while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. Distillation (as discussed in analysis:. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. chromatography involves solvent separation on a solid medium. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. Distillation takes advantage of differences in boiling points.
Ion Exchange Chromatography Presentation
Filtration Vs Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid material. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. Distillation takes advantage of differences in boiling points. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. chromatography involves solvent separation on a solid medium. Distillation (as discussed in analysis:. while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. Evaporation removes a liquid from a solution to leave a solid material. Filtration separates solids of different sizes. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by.
From www.theengineeringconcepts.com
Types of Distillation The Engineering Concepts Filtration Vs Distillation And Chromatography distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. chromatography involves solvent separation on a solid medium. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid). Filtration Vs Distillation And Chromatography.
From app.gumroad.com
GIFs for Filtration, Evaporation, Distillation and Chromatography Filtration Vs Distillation And Chromatography while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. Filtration separates solids of different sizes. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. chromatography. Filtration Vs Distillation And Chromatography.
From www.numerade.com
SOLVED 'Which methods separate a mixture according to the size of the Filtration Vs Distillation And Chromatography chromatography involves solvent separation on a solid medium. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. while they share the common goal of separation, chromatography and distillation differ in their principles, applications,. Filtration Vs Distillation And Chromatography.
From aimhigh.space
Distillation and Chromatography AimHigh! Filtration Vs Distillation And Chromatography chromatography involves solvent separation on a solid medium. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. distillation is the use of heat to separate the components of a liquid and/or gas, while. Filtration Vs Distillation And Chromatography.
From hxeyqffol.blob.core.windows.net
Filtration And Distillation Are at Jeffrey Milsap blog Filtration Vs Distillation And Chromatography chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. Filtration separates solids of different sizes. chromatography involves solvent separation on a solid medium. Distillation takes advantage of differences in boiling points. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to. Filtration Vs Distillation And Chromatography.
From studylib.net
Chromatography and distillation Filtration Vs Distillation And Chromatography Distillation takes advantage of differences in boiling points. Distillation (as discussed in analysis:. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. Filtration separates solids of different sizes. distillation is the use of heat. Filtration Vs Distillation And Chromatography.
From slidetodoc.com
Distillation and Chromatography Objectives Separate the components of Filtration Vs Distillation And Chromatography while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. Filtration separates solids of different sizes. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used. Filtration Vs Distillation And Chromatography.
From www.youtube.com
GCSE Chemistry 19 Which Separation Technique? Chromatography Filtration Vs Distillation And Chromatography distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. chromatography involves solvent separation on a solid medium. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. chromatography is a technique used to separate components of a homogenous mixture based on their affinity. Filtration Vs Distillation And Chromatography.
From www.slideserve.com
PPT Chapter 2 Properties of Matter PowerPoint Presentation, free Filtration Vs Distillation And Chromatography chromatography involves solvent separation on a solid medium. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid). Filtration Vs Distillation And Chromatography.
From fyohnrrqb.blob.core.windows.net
Liquid Chromatography Simple Explanation at Thomas Myers blog Filtration Vs Distillation And Chromatography chromatography involves solvent separation on a solid medium. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. Filtration separates solids of different sizes. while they share the common goal of separation, chromatography and distillation. Filtration Vs Distillation And Chromatography.
From video.yckmc.edu.hk
Chemistry tutorialCh621Filtration, crystallization, simple Filtration Vs Distillation And Chromatography there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. chromatography involves solvent separation on a solid medium. Filtration separates solids of different sizes. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. distillation is the use of heat to separate the components. Filtration Vs Distillation And Chromatography.
From www.teachoo.com
What are the Properties of Mixtures? Teachoo Concepts Filtration Vs Distillation And Chromatography while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. Distillation (as discussed in analysis:. Distillation takes advantage of differences in boiling points. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. Evaporation removes a liquid from a solution to leave a solid material. . Filtration Vs Distillation And Chromatography.
From slideplayer.com
Always have the same composition and are formed by chemical processes Filtration Vs Distillation And Chromatography Evaporation removes a liquid from a solution to leave a solid material. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. Distillation takes advantage of differences in boiling points. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. distillation is the use. Filtration Vs Distillation And Chromatography.
From www.slidemake.com
Ion Exchange Chromatography Presentation Filtration Vs Distillation And Chromatography distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. Evaporation removes a liquid from a solution to leave a solid material. Distillation takes advantage of differences in boiling points. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. there are different ways to. Filtration Vs Distillation And Chromatography.
From www.youtube.com
Component Separation Chromatography and Distillation YouTube Filtration Vs Distillation And Chromatography while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. Distillation takes advantage of differences in boiling points. Evaporation removes a liquid from a solution to leave a solid material. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. Filtration separates solids. Filtration Vs Distillation And Chromatography.
From fyopjkegp.blob.core.windows.net
Filtration Distillation Chromatography at Dayna Henderson blog Filtration Vs Distillation And Chromatography chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. Distillation (as discussed in analysis:. Filtration separates solids of different sizes. chromatography involves solvent separation on a solid medium. there are different ways. Filtration Vs Distillation And Chromatography.
From www.studypool.com
SOLUTION Distillation and chromatography Studypool Filtration Vs Distillation And Chromatography distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. Evaporation removes a liquid from a solution to leave a solid material. Distillation takes advantage of differences in boiling points. Distillation (as discussed in analysis:. distillation is the use of heat to separate the components of a liquid and/or gas,. Filtration Vs Distillation And Chromatography.
From mavink.com
Chromatography Diagram Labeled Filtration Vs Distillation And Chromatography chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. Distillation takes advantage of differences in boiling points. Filtration separates. Filtration Vs Distillation And Chromatography.
From dokumen.tips
(PPT) Filtration, Evaporation, Chromatography, Distillation DOKUMEN.TIPS Filtration Vs Distillation And Chromatography there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. Evaporation removes a liquid from a solution to leave a. Filtration Vs Distillation And Chromatography.
From dokumen.tips
(PPT) Distillation and Chromatography. Objectives Separate the Filtration Vs Distillation And Chromatography while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. Evaporation removes a liquid from a solution to leave a solid material. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. Distillation (as discussed in analysis:. Distillation takes advantage of differences in boiling points. . Filtration Vs Distillation And Chromatography.
From primaryleap.co.uk
Chemistry Separating Mixtures Level 1 activity for kids PrimaryLeap Filtration Vs Distillation And Chromatography there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. distillation is the use of. Filtration Vs Distillation And Chromatography.
From www.youtube.com
How to separate Mixtures filtration, distillation, sublimation and Filtration Vs Distillation And Chromatography Filtration separates solids of different sizes. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the. Filtration Vs Distillation And Chromatography.
From exyuidtbw.blob.core.windows.net
Chromatography Vs Distillation at Nancy Rogers blog Filtration Vs Distillation And Chromatography chromatography involves solvent separation on a solid medium. Filtration separates solids of different sizes. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. Distillation takes advantage of differences in boiling points. Distillation (as discussed in analysis:. while they share the common goal of separation, chromatography and distillation differ. Filtration Vs Distillation And Chromatography.
From fyowmlzue.blob.core.windows.net
Solid Liquid Chromatography at Rose Milne blog Filtration Vs Distillation And Chromatography Filtration separates solids of different sizes. Evaporation removes a liquid from a solution to leave a solid material. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. there are different ways to separate mixtures, eg. Filtration Vs Distillation And Chromatography.
From www.slideserve.com
PPT CHEMISTRY I PowerPoint Presentation, free download ID3104697 Filtration Vs Distillation And Chromatography distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. Distillation (as discussed in analysis:. Evaporation removes a liquid from a solution to leave a solid material. there are different ways to separate mixtures, eg by. Filtration Vs Distillation And Chromatography.
From www.chemicals.co.uk
GCSE Chemistry Water Purification Guide The Chemistry Blog Filtration Vs Distillation And Chromatography distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. Distillation takes advantage of differences in boiling points. chromatography involves solvent separation on a solid medium. while they share the. Filtration Vs Distillation And Chromatography.
From www.youtube.com
Organic Chemistry Class 11 Chemistry Chapter 2 Sublimation Filtration Vs Distillation And Chromatography Distillation takes advantage of differences in boiling points. Filtration separates solids of different sizes. Distillation (as discussed in analysis:. while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. distillation is the. Filtration Vs Distillation And Chromatography.
From thenoveldifference.com
Difference Between Distillation and Chromatography Filtration Vs Distillation And Chromatography chromatography involves solvent separation on a solid medium. Distillation (as discussed in analysis:. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Evaporation removes a liquid from a solution to leave a solid material. Distillation takes advantage of differences in boiling points. distillation is the selective boiling and subsequent condensation of a. Filtration Vs Distillation And Chromatography.
From www.scribd.com
Separating Mixtures Filtration, Evaporation, Distillation, and Filtration Vs Distillation And Chromatography Filtration separates solids of different sizes. Evaporation removes a liquid from a solution to leave a solid material. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. there are different ways to separate mixtures,. Filtration Vs Distillation And Chromatography.
From future4200.com
Liquid Chromatography vs. Distillation Infograph from Folium Filtration Vs Distillation And Chromatography Distillation (as discussed in analysis:. chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. Evaporation removes a liquid from a solution to leave a solid material. while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. distillation is the use of. Filtration Vs Distillation And Chromatography.
From hxeyqffol.blob.core.windows.net
Filtration And Distillation Are at Jeffrey Milsap blog Filtration Vs Distillation And Chromatography distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. Evaporation removes a liquid from a solution to leave a solid material. while they share the common goal of separation, chromatography and distillation differ in their principles, applications, and. there are different ways to separate mixtures, eg by filtration,. Filtration Vs Distillation And Chromatography.
From www.slideserve.com
PPT Distillation & Chromatography PowerPoint Presentation, free Filtration Vs Distillation And Chromatography chromatography involves solvent separation on a solid medium. distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid). Filtration Vs Distillation And Chromatography.
From www.youtube.com
Distillation and Gas Chromatography YouTube Filtration Vs Distillation And Chromatography chromatography is a technique used to separate components of a homogenous mixture based on their affinity for the. Distillation takes advantage of differences in boiling points. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration. Filtration Vs Distillation And Chromatography.
From chem.libretexts.org
1.8 Classification of Matter Chemistry LibreTexts Filtration Vs Distillation And Chromatography there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation takes advantage of differences in boiling points. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. chromatography is a. Filtration Vs Distillation And Chromatography.
From exyowdwhz.blob.core.windows.net
Chromatography A Level Chemistry Ppt at Anthony Marsden blog Filtration Vs Distillation And Chromatography distillation is the selective boiling and subsequent condensation of a component in a liquid mixture, used to separate. Distillation (as discussed in analysis:. chromatography involves solvent separation on a solid medium. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either. Filtration Vs Distillation And Chromatography.