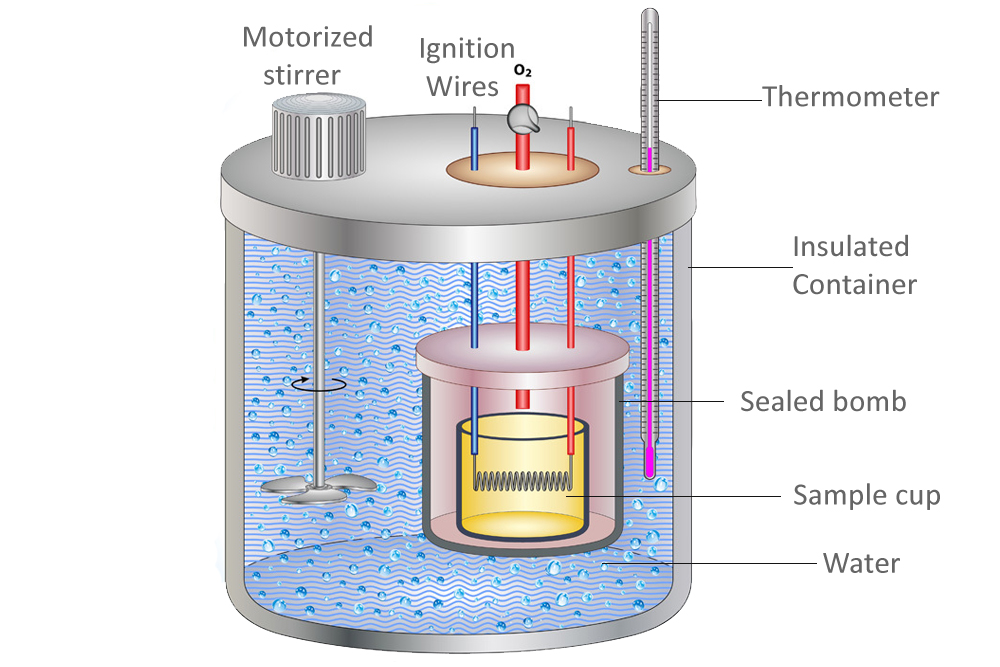
Heat Capacity Is Calorimeter Constant . what is the calorimeter constant? It's the amount of heat. that is, the calorimeter constant is the calorimeter's heat capacitance. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. We need to find the difference between the heat lost by the hot water when it droped. Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. It can analyze the heat exchange between up to 3 objects. the calorimetry calculator can help you solve complex calorimetry problems. the calibration gives you a number called the calorimeter constant. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume.
from www.scienceabc.com
that is, the calorimeter constant is the calorimeter's heat capacitance. It can analyze the heat exchange between up to 3 objects. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. the calibration gives you a number called the calorimeter constant. We need to find the difference between the heat lost by the hot water when it droped. It's the amount of heat. what is the calorimeter constant? the calorimetry calculator can help you solve complex calorimetry problems. Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter.
Molar Heat Capacity Definition, Formula, Equation, Calculation
Heat Capacity Is Calorimeter Constant It's the amount of heat. that is, the calorimeter constant is the calorimeter's heat capacitance. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. what is the calorimeter constant? the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. It's the amount of heat. the calibration gives you a number called the calorimeter constant. We need to find the difference between the heat lost by the hot water when it droped. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Heat Capacity Is Calorimeter Constant We need to find the difference between the heat lost by the hot water when it droped. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. the calorimetry calculator can help you solve complex calorimetry problems. what is the calorimeter constant? Consider. Heat Capacity Is Calorimeter Constant.
From fyogshfpe.blob.core.windows.net
Lab Calorimetry And Specific Heat Data Table at Russell Kelly blog Heat Capacity Is Calorimeter Constant Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. the calorimetry calculator can help you solve complex calorimetry problems. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant. Heat Capacity Is Calorimeter Constant.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube Heat Capacity Is Calorimeter Constant We need to find the difference between the heat lost by the hot water when it droped. the calibration gives you a number called the calorimeter constant. the calorimetry calculator can help you solve complex calorimetry problems. It's the amount of heat. Consider dropping a hot object into cold water that has been sitting in a calorimeter long. Heat Capacity Is Calorimeter Constant.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Heat Capacity Is Calorimeter Constant what is the calorimeter constant? the calibration gives you a number called the calorimeter constant. We need to find the difference between the heat lost by the hot water when it droped. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. . Heat Capacity Is Calorimeter Constant.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity Is Calorimeter Constant what is the calorimeter constant? the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. It's the amount of heat. the calibration gives you a number called the calorimeter constant. the heat capacity of the calorimeter or of the reaction mixture may. Heat Capacity Is Calorimeter Constant.
From www.youtube.com
Principle of Calorimetry YouTube Heat Capacity Is Calorimeter Constant that is, the calorimeter constant is the calorimeter's heat capacitance. the calorimetry calculator can help you solve complex calorimetry problems. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. It can analyze the heat exchange between up to 3 objects. It's the. Heat Capacity Is Calorimeter Constant.
From giouwhids.blob.core.windows.net
Calorimeter Definition Oxford at Lisa Dinsmore blog Heat Capacity Is Calorimeter Constant It's the amount of heat. Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. It can analyze the heat exchange between up to 3 objects. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure. Heat Capacity Is Calorimeter Constant.
From lessonlibpigmentary.z13.web.core.windows.net
How To Calculate A Specific Heat Heat Capacity Is Calorimeter Constant the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. the calibration gives you a number called the calorimeter constant. what is the calorimeter constant? It's the amount of heat. We need to find the difference between the heat lost by the hot. Heat Capacity Is Calorimeter Constant.
From exychpdvn.blob.core.windows.net
Why Is It Important To Determine The Heat Capacity Of The Calorimeter Heat Capacity Is Calorimeter Constant We need to find the difference between the heat lost by the hot water when it droped. that is, the calorimeter constant is the calorimeter's heat capacitance. the calibration gives you a number called the calorimeter constant. It's the amount of heat. the heat capacity of the calorimeter or of the reaction mixture may be used to. Heat Capacity Is Calorimeter Constant.
From exychpdvn.blob.core.windows.net
Why Is It Important To Determine The Heat Capacity Of The Calorimeter Heat Capacity Is Calorimeter Constant We need to find the difference between the heat lost by the hot water when it droped. It's the amount of heat. the calorimetry calculator can help you solve complex calorimetry problems. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. the. Heat Capacity Is Calorimeter Constant.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Heat Capacity Is Calorimeter Constant We need to find the difference between the heat lost by the hot water when it droped. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. It's the amount of heat. the heat capacity of the calorimeter or of the reaction mixture may. Heat Capacity Is Calorimeter Constant.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Heat Capacity Is Calorimeter Constant the calibration gives you a number called the calorimeter constant. what is the calorimeter constant? the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. that is, the calorimeter constant is the calorimeter's heat capacitance. It's the amount of heat.. Heat Capacity Is Calorimeter Constant.
From fyocdslfs.blob.core.windows.net
Calorimetry Explained at Edith Wallis blog Heat Capacity Is Calorimeter Constant the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. what is the calorimeter constant? the calibration gives you a number called the calorimeter constant. It's the amount of heat. that is, the calorimeter constant is the calorimeter's heat capacitance. the. Heat Capacity Is Calorimeter Constant.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity Is Calorimeter Constant Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. the calorimetry calculator can help you solve complex calorimetry problems. what is the calorimeter constant? It's the amount of heat. the heat capacity ratio (or adiabatic index ) is the ratio of. Heat Capacity Is Calorimeter Constant.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Heat Capacity Is Calorimeter Constant It's the amount of heat. It can analyze the heat exchange between up to 3 objects. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at. Heat Capacity Is Calorimeter Constant.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Heat Capacity Is Calorimeter Constant what is the calorimeter constant? the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. It's the amount of heat. We need to find the difference between the heat lost by the hot water when it droped. that is, the calorimeter constant is. Heat Capacity Is Calorimeter Constant.
From exyqpgrbs.blob.core.windows.net
Calorimetry Equation Explained at Sandra Roof blog Heat Capacity Is Calorimeter Constant the calorimetry calculator can help you solve complex calorimetry problems. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity. Heat Capacity Is Calorimeter Constant.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Heat Capacity Is Calorimeter Constant what is the calorimeter constant? Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. We need to find the difference between the heat lost by the hot water when it droped. the calibration gives you a number called the calorimeter constant. . Heat Capacity Is Calorimeter Constant.
From askfilo.com
In a constant pressure calorimeter with heat capacity of 453 Jk−1,200 mL Heat Capacity Is Calorimeter Constant It's the amount of heat. what is the calorimeter constant? that is, the calorimeter constant is the calorimeter's heat capacitance. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. We need to find the difference between the heat lost by the hot water when it. Heat Capacity Is Calorimeter Constant.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Heat Capacity Is Calorimeter Constant It can analyze the heat exchange between up to 3 objects. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. the calorimetry calculator can help you solve complex calorimetry problems. Consider dropping a hot object into cold water that has been sitting in. Heat Capacity Is Calorimeter Constant.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Capacity Is Calorimeter Constant the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. what is the calorimeter constant? the calibration gives you a number called the calorimeter constant. Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to. Heat Capacity Is Calorimeter Constant.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Heat Capacity Is Calorimeter Constant the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. It's the amount of heat. that is, the calorimeter constant is the calorimeter's heat capacitance. the calorimetry calculator can help you solve complex calorimetry problems. what is the calorimeter constant?. Heat Capacity Is Calorimeter Constant.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity Is Calorimeter Constant the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. the calorimetry calculator can help you solve complex calorimetry problems. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity. Heat Capacity Is Calorimeter Constant.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity Is Calorimeter Constant Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. that is, the calorimeter constant is the calorimeter's heat capacitance. the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. the. Heat Capacity Is Calorimeter Constant.
From giouwhids.blob.core.windows.net
Calorimeter Definition Oxford at Lisa Dinsmore blog Heat Capacity Is Calorimeter Constant what is the calorimeter constant? Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. It can analyze the heat exchange between up to 3 objects. that is, the calorimeter constant is the calorimeter's heat capacitance. the calibration gives you a number. Heat Capacity Is Calorimeter Constant.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Heat Capacity Is Calorimeter Constant We need to find the difference between the heat lost by the hot water when it droped. what is the calorimeter constant? It can analyze the heat exchange between up to 3 objects. Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. . Heat Capacity Is Calorimeter Constant.
From giojclhhk.blob.core.windows.net
How To Calculate Calorimeter Constant Problems at Michael Massie blog Heat Capacity Is Calorimeter Constant that is, the calorimeter constant is the calorimeter's heat capacitance. It can analyze the heat exchange between up to 3 objects. We need to find the difference between the heat lost by the hot water when it droped. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat. Heat Capacity Is Calorimeter Constant.
From chempedia.info
Constants for heat capacity Big Chemical Encyclopedia Heat Capacity Is Calorimeter Constant what is the calorimeter constant? the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. that is, the calorimeter constant is the calorimeter's heat capacitance. the calibration gives you a number called the calorimeter constant. the heat capacity ratio. Heat Capacity Is Calorimeter Constant.
From hxemseydn.blob.core.windows.net
How To Calculate Heat Of Calorimeter at Lucille Monahan blog Heat Capacity Is Calorimeter Constant the calorimetry calculator can help you solve complex calorimetry problems. We need to find the difference between the heat lost by the hot water when it droped. It can analyze the heat exchange between up to 3 objects. the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat. Heat Capacity Is Calorimeter Constant.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Heat Capacity Is Calorimeter Constant Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. that is, the calorimeter constant is the calorimeter's heat capacitance. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. Heat Capacity Is Calorimeter Constant.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Heat Capacity Is Calorimeter Constant the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. that is, the calorimeter constant is the calorimeter's heat capacitance. Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the. Heat Capacity Is Calorimeter Constant.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Heat Capacity Is Calorimeter Constant the heat capacity ratio (or adiabatic index ) is the ratio of the heat capacity at constant pressure to heat capacity at constant volume. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. the calibration gives you a number called. Heat Capacity Is Calorimeter Constant.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Heat Capacity Is Calorimeter Constant the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. the calibration gives you a number called the calorimeter constant. It's the amount of heat. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released. Heat Capacity Is Calorimeter Constant.
From www.youtube.com
How to find calorimeter constant YouTube Heat Capacity Is Calorimeter Constant what is the calorimeter constant? the calorimetry calculator can help you solve complex calorimetry problems. Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the. Heat Capacity Is Calorimeter Constant.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Heat Capacity Is Calorimeter Constant Consider dropping a hot object into cold water that has been sitting in a calorimeter long enough to have the same temperature as the calorimeter. the calorimetry calculator can help you solve complex calorimetry problems. what is the calorimeter constant? It can analyze the heat exchange between up to 3 objects. It's the amount of heat. We need. Heat Capacity Is Calorimeter Constant.