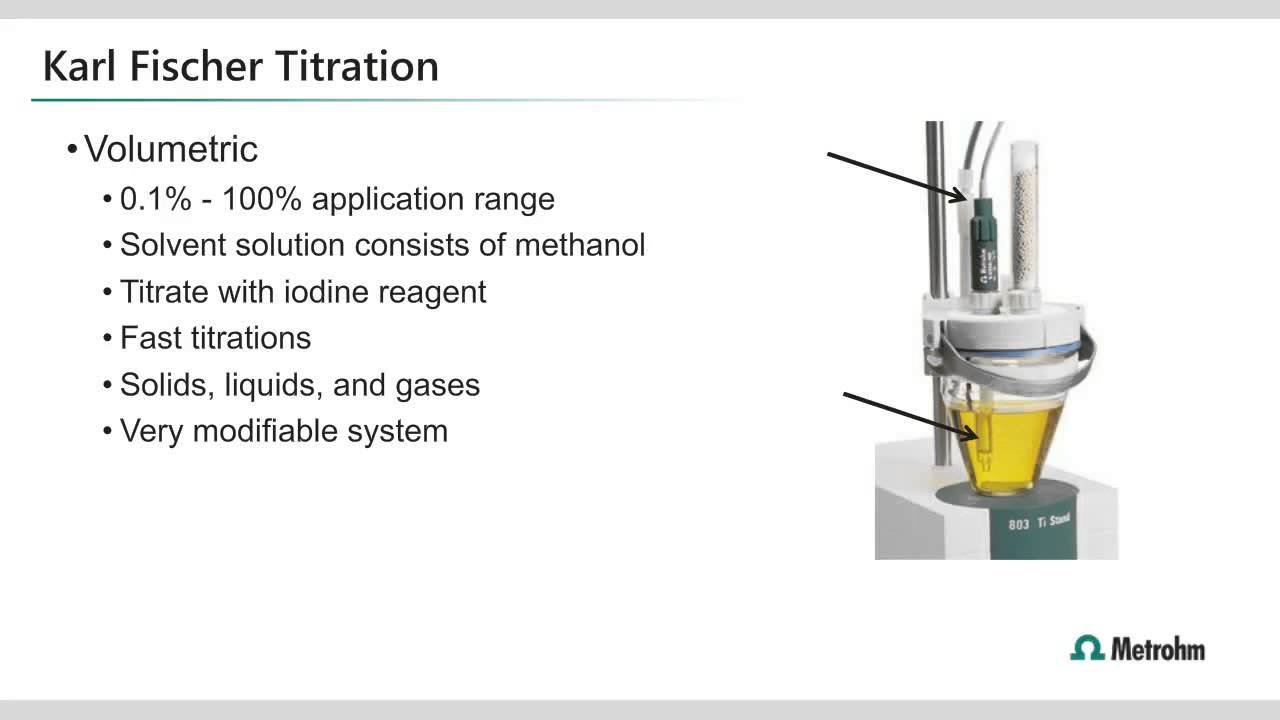
Principle Titration Technique . A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. This titration technique relies on a chemical. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Redox titrations involve the transfer of electrons from one species to another.
from www.youtube.com
Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Redox titrations involve the transfer of electrons from one species to another. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of. This titration technique relies on a chemical. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances.
Moisture Analysis in Pharma Expanding Near Infrared Spectroscopy with
Principle Titration Technique Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Redox titrations involve the transfer of electrons from one species to another. This titration technique relies on a chemical. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of.
From present5.com
Titration and AcidBase Neutralization LEARNING OBJECTIVES 11.2.2.7 Principle Titration Technique Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a technique of determining the concentration of an unknown solution by using a solution of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Principle Titration Technique.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Principle Titration Technique This titration technique relies on a chemical. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Titration is the slow addition of one solution of a. Principle Titration Technique.
From www.slideshare.net
Titration principle, working and application Principle Titration Technique A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Principle Titration Technique.
From gamma.app
Understanding Titration Principle Titration Technique Redox titrations involve the transfer of electrons from one species to another. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is a. Principle Titration Technique.
From www.slideshare.net
Titration principle, working and application Principle Titration Technique Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Redox titrations involve the transfer of electrons from one species to another. This titration technique relies on. Principle Titration Technique.
From www.sliderbase.com
Titration Principle Titration Technique A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Titration is the slow addition of one solution of a known. Principle Titration Technique.
From mcckf.com
What is the Karl Fischer Method? Karl Fischer Method AQUAMICRON by Principle Titration Technique Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. Titration is a technique of determining the concentration of an unknown solution. Principle Titration Technique.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Principle Titration Technique A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. This titration technique relies on a chemical. Redox titrations involve the transfer of electrons from. Principle Titration Technique.
From www.youtube.com
Basic Principles, Methods & Application of Diazotization Titration Principle Titration Technique Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This titration technique relies on a chemical. A titration is the process of determining the quantity of. Principle Titration Technique.
From www.youtube.com
Moisture Analysis in Pharma Expanding Near Infrared Spectroscopy with Principle Titration Technique A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is the process of determining the quantity of a substance a by adding. Principle Titration Technique.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Principle Titration Technique A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. Principle Titration Technique.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Principle Titration Technique A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content. Principle Titration Technique.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 Principle Titration Technique A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to. Principle Titration Technique.
From myleadtracker.com
titration practical Principle Titration Technique Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Principle Titration Technique.
From chemistnotes.com
Karl Fischer Titration easy principle, 2 types, advantages Principle Titration Technique Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. Principle Titration Technique.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Principle Titration Technique Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Redox titrations involve the transfer of electrons from one species to another. Titration is the slow addition. Principle Titration Technique.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Principle Titration Technique This titration technique relies on a chemical. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Titration is a technique of determining the concentration of an unknown solution by using a solution of. A titration is the process of determining the quantity of a. Principle Titration Technique.
From byjus.com
What is the Karl Fischer Method? Principle, Method, Applications Principle Titration Technique Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. A titration is a laboratory technique used to precisely measure molar concentration. Principle Titration Technique.
From www.brainkart.com
Amperometric Methods Instrumentation Principle Titration Technique Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Redox titrations involve the transfer of electrons from one species to another. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it.. Principle Titration Technique.
From www.youtube.com
Precipitation Titrations Principle Mohr’s method Volhard’s Principle Titration Technique This titration technique relies on a chemical. Titration is a technique of determining the concentration of an unknown solution by using a solution of. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. A titration is a laboratory technique used to precisely measure molar concentration. Principle Titration Technique.
From www.creative-biostructure.com
Isothermal Titration Calorimetry (ITC) for Coronavirus Research Principle Titration Technique This titration technique relies on a chemical. Titration is a technique of determining the concentration of an unknown solution by using a solution of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Redox titrations involve the transfer of electrons from one species to another.. Principle Titration Technique.
From www.zogirls.com
Performing a Titration & Volumetric Analysis (4.1.2) AQA A Level Principle Titration Technique Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is the process of determining the quantity of a substance. Principle Titration Technique.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Principle Titration Technique Titration is a technique of determining the concentration of an unknown solution by using a solution of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of. Principle Titration Technique.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Principle Titration Technique This titration technique relies on a chemical. Titration is a technique of determining the concentration of an unknown solution by using a solution of. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Karl fischer titration is a widely utilized analytical method specifically designed. Principle Titration Technique.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Principle Titration Technique A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This titration technique relies on a chemical. Titration is the slow addition of one solution of. Principle Titration Technique.
From letitsnowglobe.co.uk
Titration procedure pdf Principle Titration Technique Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Redox titrations involve the transfer of electrons from one species to. Principle Titration Technique.
From www.slideserve.com
PPT Lecture Date March 26 th , 2012 PowerPoint Presentation, free Principle Titration Technique Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. This titration technique relies on a chemical. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is the process. Principle Titration Technique.
From www.studyread.com
Potentiometric Titration with Its Principle, Applications and Advanatages Principle Titration Technique A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Redox titrations involve the transfer of electrons from one species to another. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration, process of chemical analysis in which the quantity. Principle Titration Technique.
From www.metrohm.com
What to consider during backtitration Metrohm Principle Titration Technique Titration is a technique of determining the concentration of an unknown solution by using a solution of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances.. Principle Titration Technique.
From theedge.com.hk
Chemistry How To Titration The Edge Principle Titration Technique Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is a laboratory technique used to precisely measure molar. Principle Titration Technique.
From mungfali.com
Titration Steps Principle Titration Technique A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. Redox titrations involve the transfer of electrons from one species to another. This titration technique relies on a chemical. Titration is the slow addition of one solution of a known concentration (called a titrant) to a. Principle Titration Technique.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Principle Titration Technique Titration, also known as titrimetry, is a chemical qualitative analysis technique that is used to calculate the concentration of a given analyte in a mixture. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which it. This titration technique relies on a chemical. Titration, process of chemical. Principle Titration Technique.
From www.slideserve.com
PPT Principles of Volumetric (Titrimetric) Analysis PowerPoint Principle Titration Technique A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique of determining the concentration of an unknown solution by using a solution of. A titration is the process of determining the quantity of a substance a by adding measured increments of substance b , with which. Principle Titration Technique.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Principle Titration Technique Titration is a technique of determining the concentration of an unknown solution by using a solution of. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. This titration technique relies on a chemical. Titration, also known as titrimetry, is a chemical qualitative analysis technique that. Principle Titration Technique.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Principle Titration Technique Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. Principle Titration Technique.