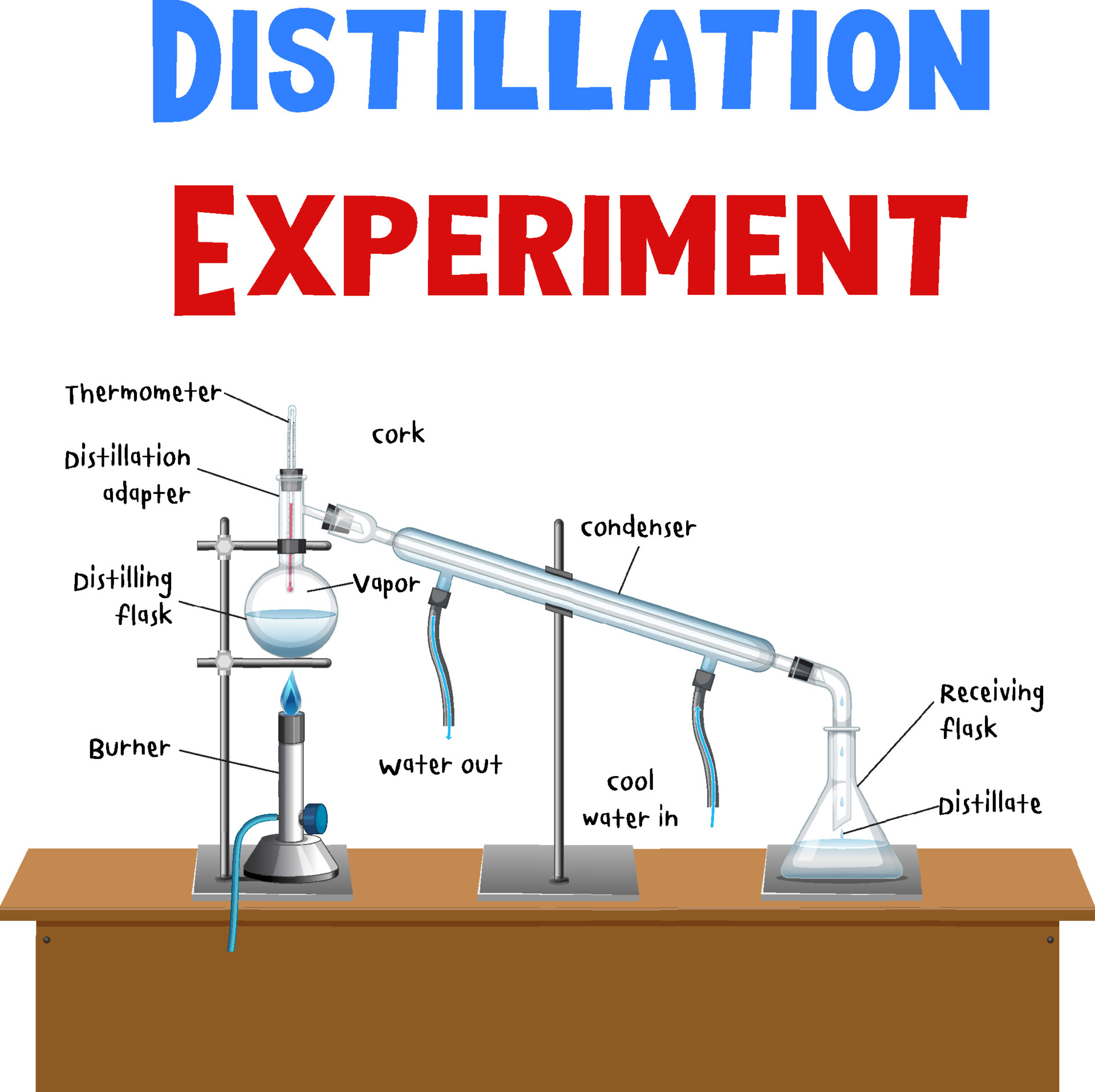
Results Of Distillation Experiment . Under simple distillation, this means that the lower boiling point component has already finished distilling. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Ing vapor is the basis of distillation. In this lab tutorial, we discuss simple distillation, its difference from fractional. An appropriate distillation collects distillate at a rate of 1 drop per second. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. Organic liquids containing small amounts (<15%) of impurities. An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances.
from www.vecteezy.com
An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. Organic liquids containing small amounts (<15%) of impurities. An appropriate distillation collects distillate at a rate of 1 drop per second. Under simple distillation, this means that the lower boiling point component has already finished distilling. In this lab tutorial, we discuss simple distillation, its difference from fractional. Ing vapor is the basis of distillation. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points.
Science experiment with distillation 6763633 Vector Art at Vecteezy
Results Of Distillation Experiment In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Ing vapor is the basis of distillation. An appropriate distillation collects distillate at a rate of 1 drop per second. In this lab tutorial, we discuss simple distillation, its difference from fractional. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. Organic liquids containing small amounts (<15%) of impurities. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Under simple distillation, this means that the lower boiling point component has already finished distilling. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points.
From speichim.com
Main principes of distillation Speichim Processing Valls Química Results Of Distillation Experiment An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. Under simple distillation, this means that the lower boiling point component has already finished distilling. Organic liquids containing small amounts (<15%) of impurities. Ing vapor is the basis of distillation. An appropriate distillation collects distillate at a rate of. Results Of Distillation Experiment.
From chem.libretexts.org
3.6 Changes in Matter Physical and Chemical Changes Chemistry Results Of Distillation Experiment An appropriate distillation collects distillate at a rate of 1 drop per second. An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. Under simple distillation, this means that the lower. Results Of Distillation Experiment.
From studyrocket.co.uk
Distillation GCSE Chemistry Science) OCR Revision Study Results Of Distillation Experiment Organic liquids containing small amounts (<15%) of impurities. An appropriate distillation collects distillate at a rate of 1 drop per second. Ing vapor is the basis of distillation. An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. Distillation is a purification method for liquids, and can separate components. Results Of Distillation Experiment.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Results Of Distillation Experiment Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. Under simple distillation, this means that the lower boiling point component has already finished distilling. An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. From a graph of temperature vs. Results Of Distillation Experiment.
From www.youtube.com
Introduction to Fractional distillation Distillation procedure Home Results Of Distillation Experiment An appropriate distillation collects distillate at a rate of 1 drop per second. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Organic liquids containing small amounts (<15%) of impurities. An example of a simple distillation is the separation of a solution of salt and water into two. Results Of Distillation Experiment.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Results Of Distillation Experiment An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. Under simple distillation, this means that the lower boiling point component has already finished distilling. An appropriate distillation collects distillate at. Results Of Distillation Experiment.
From www.slideshare.net
Experiment 1 Distillation of Alcoholic Beverages Results Of Distillation Experiment In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. An appropriate distillation collects distillate at a rate of 1 drop per second. Organic liquids containing small amounts (<15%) of impurities. From a graph of temperature vs the total volume of distillate collected for the distillation of the. Results Of Distillation Experiment.
From www.numerade.com
SOLVED A student did a distillation experiment using 40 aqueous Results Of Distillation Experiment In this lab tutorial, we discuss simple distillation, its difference from fractional. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. An appropriate distillation collects distillate at. Results Of Distillation Experiment.
From testbook.com
team Distillation Principle, Working, Advantages & Application Results Of Distillation Experiment Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Organic liquids containing small amounts (<15%) of impurities. An appropriate distillation collects distillate at a rate of 1 drop per second. In this lab tutorial, we discuss simple distillation, its difference from fractional. Ing vapor is the basis of. Results Of Distillation Experiment.
From www.vecteezy.com
Science experiment with distillation 6763633 Vector Art at Vecteezy Results Of Distillation Experiment In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. An appropriate distillation collects distillate at a rate of 1 drop per second. Distilling at. Results Of Distillation Experiment.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Results Of Distillation Experiment Organic liquids containing small amounts (<15%) of impurities. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. In this lab tutorial, we discuss simple distillation, its difference from fractional.. Results Of Distillation Experiment.
From studylib.net
Simple Distillation Results Of Distillation Experiment Under simple distillation, this means that the lower boiling point component has already finished distilling. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. In this lab tutorial, we discuss simple distillation, its difference from fractional. An appropriate distillation collects distillate at a rate of 1. Results Of Distillation Experiment.
From www.circuitdiagram.co
Schematic Diagram Of Simple Distillation Experiment Circuit Diagram Results Of Distillation Experiment An appropriate distillation collects distillate at a rate of 1 drop per second. Organic liquids containing small amounts (<15%) of impurities. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. An example of a simple distillation is the separation of a solution of salt and water into. Results Of Distillation Experiment.
From ar.inspiredpencil.com
Distillation Diagram For Kids Results Of Distillation Experiment In this lab tutorial, we discuss simple distillation, its difference from fractional. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Under simple distillation, this means that the lower boiling point component has already finished distilling. Organic liquids containing small amounts (<15%) of impurities. Ing vapor is. Results Of Distillation Experiment.
From www.vernier.com
Fractional Distillation > Experiment 8 from Chemistry with Vernier Results Of Distillation Experiment An appropriate distillation collects distillate at a rate of 1 drop per second. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. An example of a. Results Of Distillation Experiment.
From www.thoughtco.com
What Is Distillation? Principles and Uses Results Of Distillation Experiment Ing vapor is the basis of distillation. An appropriate distillation collects distillate at a rate of 1 drop per second. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and.. Results Of Distillation Experiment.
From www.youtube.com
DISTILLATION OF WATER AND ETHANOL YouTube Results Of Distillation Experiment An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. Organic liquids containing small amounts (<15%) of impurities. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. From a graph of temperature vs the total volume. Results Of Distillation Experiment.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Results Of Distillation Experiment Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. In this lab tutorial, we discuss simple distillation, its difference from fractional. Under simple distillation, this means that the lower boiling point component has already finished distilling. An appropriate distillation collects distillate at a rate of 1 drop per second. In this. Results Of Distillation Experiment.
From studylib.net
Steam Distillation Experiment Results Of Distillation Experiment In this lab tutorial, we discuss simple distillation, its difference from fractional. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Ing vapor is the basis. Results Of Distillation Experiment.
From www.slideserve.com
PPT Experiment 10 PowerPoint Presentation, free download ID3645330 Results Of Distillation Experiment Organic liquids containing small amounts (<15%) of impurities. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. In this lab tutorial, we discuss simple distillation, its difference from fractional. Ing vapor is the basis of distillation. In this experiment, you will be conducting both a simple. Results Of Distillation Experiment.
From www.bbc.co.uk
Distillation BBC Bitesize Results Of Distillation Experiment From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. An appropriate distillation collects distillate at a rate of 1 drop per second. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Ing vapor is. Results Of Distillation Experiment.
From www.chegg.com
A. Distillation Procedure In this experiment, you Results Of Distillation Experiment In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. Ing vapor is the basis of distillation. In this lab tutorial, we discuss simple distillation,. Results Of Distillation Experiment.
From www.circuitdiagram.co
Schematic Diagram Of Simple Distillation Experiment Circuit Diagram Results Of Distillation Experiment In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Organic liquids containing small amounts (<15%) of impurities. Under simple distillation, this means that the lower boiling point component has already finished distilling. An example of a simple distillation is the separation of a solution of salt and. Results Of Distillation Experiment.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Results Of Distillation Experiment Ing vapor is the basis of distillation. In this lab tutorial, we discuss simple distillation, its difference from fractional. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. Distillation is. Results Of Distillation Experiment.
From www.researchgate.net
Schematic diagram of the membrane distillation experiment setup Results Of Distillation Experiment Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. Ing vapor is the basis of distillation. Under simple distillation, this means that the lower boiling point component has already finished distilling. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the. Results Of Distillation Experiment.
From www.researchgate.net
Experimental results for assisted imaginarity distillation. (a) Initial Results Of Distillation Experiment Under simple distillation, this means that the lower boiling point component has already finished distilling. An appropriate distillation collects distillate at a rate of 1 drop per second. Organic liquids containing small amounts (<15%) of impurities. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. In. Results Of Distillation Experiment.
From www.chegg.com
Solved STEAM DISTILLATION The purpose of this experiment is Results Of Distillation Experiment Under simple distillation, this means that the lower boiling point component has already finished distilling. In this lab tutorial, we discuss simple distillation, its difference from fractional. An appropriate distillation collects distillate at a rate of 1 drop per second. Ing vapor is the basis of distillation. An example of a simple distillation is the separation of a solution of. Results Of Distillation Experiment.
From www.nagwa.com
Lesson Distillation Nagwa Results Of Distillation Experiment Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. An appropriate distillation collects distillate at a rate of 1 drop per second. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. Distilling at too. Results Of Distillation Experiment.
From www.youtube.com
Separation Of Two Miscible Liquids By Distillation Diagram CBSE Results Of Distillation Experiment Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. An example of a simple distillation is the separation of a solution of salt and water into two separate pure substances. Ing vapor is the basis of distillation. An appropriate distillation collects distillate at a rate of 1 drop per second. Distillation. Results Of Distillation Experiment.
From www.youtube.com
ORGANIC CHEMISTRY EXPERIMENT 3 Distillation Technique YouTube Results Of Distillation Experiment Ing vapor is the basis of distillation. An appropriate distillation collects distillate at a rate of 1 drop per second. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. An example of a simple distillation is the separation of a solution of salt and water into two. Results Of Distillation Experiment.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Results Of Distillation Experiment Ing vapor is the basis of distillation. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Under simple distillation, this means that the lower boiling point component has already finished distilling. An appropriate distillation collects distillate at a rate of 1 drop per second. From a graph. Results Of Distillation Experiment.
From www.docsity.com
Simple and fractional distillation lab report Study Guides, Projects Results Of Distillation Experiment From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. Under simple distillation, this means that the lower boiling point component has already finished distilling. Ing vapor is the basis of distillation. Organic liquids containing small amounts (<15%) of impurities. Distilling at too fast a rate prevents. Results Of Distillation Experiment.
From www.vernier.com
Fractional Distillation of Esters > Experiment 9 from Organic Chemistry Results Of Distillation Experiment Organic liquids containing small amounts (<15%) of impurities. An appropriate distillation collects distillate at a rate of 1 drop per second. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results. Results Of Distillation Experiment.
From www.chegg.com
Solved EXPERIMENT 6 Simple and Fractional Distillation Results Of Distillation Experiment Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. In this lab tutorial, we discuss simple distillation, its difference from fractional. An example of a. Results Of Distillation Experiment.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Results Of Distillation Experiment From a graph of temperature vs the total volume of distillate collected for the distillation of the mixture, estimate the composition of the. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. Under simple distillation, this means that the lower boiling point component has already finished distilling. Ing vapor is the. Results Of Distillation Experiment.