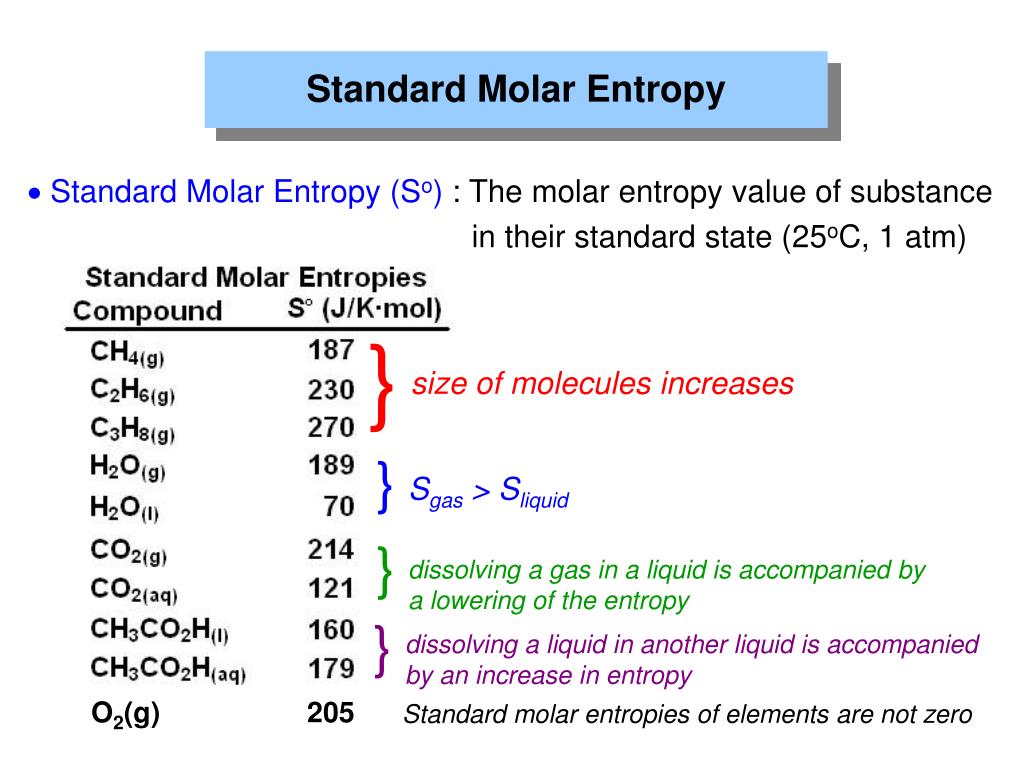
Do Bigger Molecules Have More Entropy . If you have more than. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). Notice that there isn't very big jump in. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or.
from www.slideserve.com
Notice that there isn't very big jump in. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. If you have more than. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas.
PPT Chemistry 101 Chap. 19 PowerPoint Presentation, free download
Do Bigger Molecules Have More Entropy If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). If you have more than. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. Notice that there isn't very big jump in. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy.
From slideplayer.com
Chapter 19 Thermodynamics ppt download Do Bigger Molecules Have More Entropy If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Chapter 9 Molecular Geometry and Bonding Theories PowerPoint Do Bigger Molecules Have More Entropy Notice that there isn't very big jump in. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. The. Do Bigger Molecules Have More Entropy.
From slideplayer.com
From Thermochemistry to Thermodynamics ppt download Do Bigger Molecules Have More Entropy The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and. Do Bigger Molecules Have More Entropy.
From study.com
Entropy in Chemistry Definition & Calculation Lesson Do Bigger Molecules Have More Entropy Notice that there isn't very big jump in. Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. The molecules of solids, liquids, and gases have. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Entropy Randomness & Disorder PowerPoint Presentation ID6039529 Do Bigger Molecules Have More Entropy The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. Soft crystalline substances and those with larger atoms tend to have higher entropies. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Chemical Thermodynamics PowerPoint Presentation, free download Do Bigger Molecules Have More Entropy The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. For a given substance, s. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Chapter 19 PowerPoint Presentation, free download ID6543229 Do Bigger Molecules Have More Entropy Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. If you have more than. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of. Do Bigger Molecules Have More Entropy.
From slideplayer.com
Energy Thermodynamics ppt download Do Bigger Molecules Have More Entropy If you have more than. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. Soft crystalline substances and those with larger atoms. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Chapter 19 Entropy and Free Energy PowerPoint Presentation, free Do Bigger Molecules Have More Entropy Notice that there isn't very big jump in. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. If you have more than. Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. Entropies of large, complicated molecules are greater than. Do Bigger Molecules Have More Entropy.
From jackwestin.com
Second Law Concept Of Entropy Energy Changes In Chemical Reactions Do Bigger Molecules Have More Entropy The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. Notice that there isn't very big jump in. If you have more than. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. When a liquid vaporizes, the restrictions on. Do Bigger Molecules Have More Entropy.
From scienceinfo.com
Entropy Definition, Properties, and Facts Do Bigger Molecules Have More Entropy Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. Notice that there isn't very big jump in. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Gibbs Free Energy PowerPoint Presentation, free download ID2198185 Do Bigger Molecules Have More Entropy Notice that there isn't very big jump in. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Is this your room/ PowerPoint Presentation, free download ID Do Bigger Molecules Have More Entropy If you have more than. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. The entropy increases as the molecules become more. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free Do Bigger Molecules Have More Entropy If you have more than. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. The molecules of. Do Bigger Molecules Have More Entropy.
From www.numerade.com
SOLVED Derive an expression for the molar entropy of an equally spaced Do Bigger Molecules Have More Entropy The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. When a liquid vaporizes, the restrictions on. Do Bigger Molecules Have More Entropy.
From slideplayer.com
Chapter 16 Reaction Energy ppt download Do Bigger Molecules Have More Entropy Notice that there isn't very big jump in. Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. Entropies of large,. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Chemistry 101 Chap. 19 PowerPoint Presentation, free download Do Bigger Molecules Have More Entropy The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. Entropies. Do Bigger Molecules Have More Entropy.
From slideplayer.com
From Thermochemistry to Thermodynamics ppt download Do Bigger Molecules Have More Entropy If you have more than. Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. For a given substance, s solid < s liquid <. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Chapter 18 Entropy, Free Energy, and Equilibrium Part I Do Bigger Molecules Have More Entropy Notice that there isn't very big jump in. Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. When a liquid vaporizes, the restrictions on. Do Bigger Molecules Have More Entropy.
From opentextbc.ca
Measuring Entropy and Entropy Changes Introductory Chemistry 1st Do Bigger Molecules Have More Entropy For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. The molecules of solids, liquids, and gases have increasingly greater freedom to move. Do Bigger Molecules Have More Entropy.
From www.numerade.com
SOLVED Question 34 5 pts Why does the entropy = decrease when gaseous Do Bigger Molecules Have More Entropy Notice that there isn't very big jump in. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. Entropies of large, complicated molecules are greater than those of smaller,. Do Bigger Molecules Have More Entropy.
From www.researchgate.net
figure shows that the AMET(4) molecule has a larger entropy as the Do Bigger Molecules Have More Entropy The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. Soft crystalline substances and those with larger atoms tend to have higher entropies. Do Bigger Molecules Have More Entropy.
From www.chemistrystudent.com
Entropy (ALevel) ChemistryStudent Do Bigger Molecules Have More Entropy The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. If you have more than. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. For a given substance, s solid < s liquid < s gas in a given. Do Bigger Molecules Have More Entropy.
From www.numerade.com
SOLVED The entropy will usually increase when L.a molecule is broken Do Bigger Molecules Have More Entropy Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. If you have more than. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. The entropy increases as the molecules become more disordered as you go from. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Chapter 19 Thermodynamics PowerPoint Presentation, free download Do Bigger Molecules Have More Entropy If you have more than. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. Soft crystalline substances and those with larger atoms. Do Bigger Molecules Have More Entropy.
From slideplayer.com
Chapter 19 Free Energy and Thermodynamics ppt download Do Bigger Molecules Have More Entropy For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. Soft crystalline substances and those with larger atoms tend to have higher. Do Bigger Molecules Have More Entropy.
From courses.lumenlearning.com
Entropy Chemistry Atoms First Do Bigger Molecules Have More Entropy Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. The. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Thermodynamics Entropy, Energy and equilibrium PowerPoint Do Bigger Molecules Have More Entropy The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. When a liquid vaporizes, the restrictions on the molecules’ ability to move around are relaxed almost completely and a. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. Entropies. Do Bigger Molecules Have More Entropy.
From www.pinterest.ca
What is Entropy ' A measurement of the degree of randomness of energy Do Bigger Molecules Have More Entropy Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy is typically greater for heavier atoms or. If you only have one particle, then that system of one particle can subsist in two. Do Bigger Molecules Have More Entropy.
From www.mdpi.com
Entropy Free FullText Thermodynamics Beyond Molecules Statistical Do Bigger Molecules Have More Entropy Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. Entropies of large, complicated molecules are greater than those of smaller, simpler molecules (column 2). The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. For a given substance, s solid <. Do Bigger Molecules Have More Entropy.
From slideplayer.com
Chemical Thermodynamics ppt download Do Bigger Molecules Have More Entropy The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. Notice that there isn't very big jump in. Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. If you have more than. Entropies of large, complicated molecules are greater than those. Do Bigger Molecules Have More Entropy.
From www.slideserve.com
PPT Chapter 17 Free Energy and Thermodynamics PowerPoint Presentation Do Bigger Molecules Have More Entropy If you have more than. The entropy increases as the molecules become more disordered as you go from solid to liquid to gas. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. The molecules of solids, liquids, and gases have increasingly greater freedom to. Do Bigger Molecules Have More Entropy.
From www.numerade.com
SOLVEDWrite out the equation for free energy as a function of enthalpy Do Bigger Molecules Have More Entropy Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. The entropy increases as the molecules become more disordered as you go from solid to. Do Bigger Molecules Have More Entropy.
From slideplayer.com
From Thermochemistry to Thermodynamics ppt download Do Bigger Molecules Have More Entropy If you only have one particle, then that system of one particle can subsist in two states, one side of the box versus the other. If you have more than. Notice that there isn't very big jump in. For a given substance, s solid < s liquid < s gas in a given physical state at a given temperature, entropy. Do Bigger Molecules Have More Entropy.
From sciencenotes.org
What Is Entropy? Definition and Examples Do Bigger Molecules Have More Entropy Notice that there isn't very big jump in. Soft crystalline substances and those with larger atoms tend to have higher entropies because of increased molecular motion and disorder. The molecules of solids, liquids, and gases have increasingly greater freedom to move around, facilitating the spreading and sharing of thermal energy. If you only have one particle, then that system of. Do Bigger Molecules Have More Entropy.