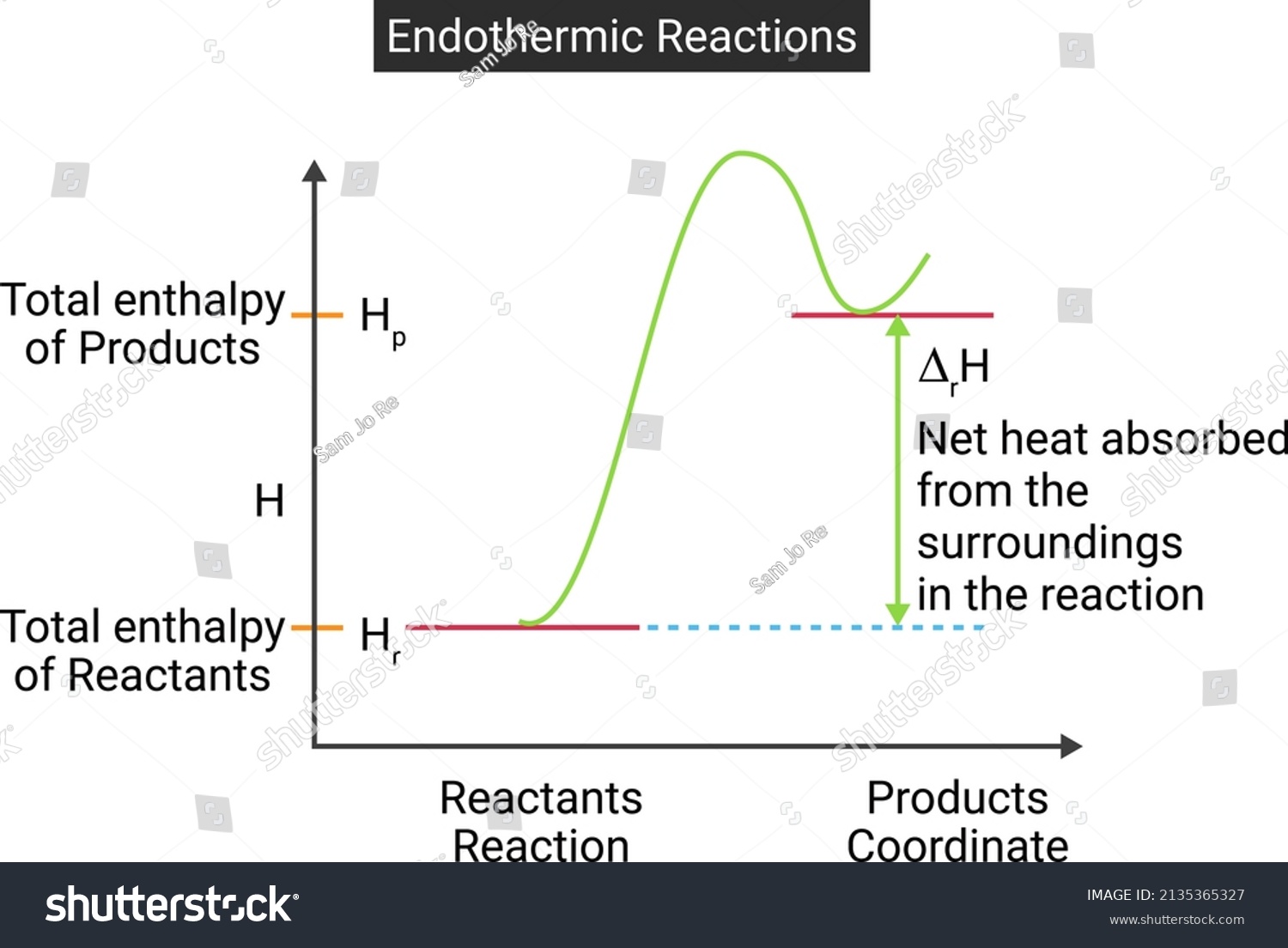
Endothermic Enthalpy Of Solution . \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. By the end of this section, you will be able to: The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. if the energy required to break apart the lattice is greater than the energy released by hydration, enthalpy of solution is endothermic (+δh). the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. Use bond dissociation energies to calculate enthalpy change or heat of reaction. If the energy required to break apart the lattice is smaller than the. State the first law of thermodynamics. Key takeaway enthalpy is a state function. the enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution. the enthalpy of solution can expressed as the sum of enthalpy changes for each step: Determine if a chemical process is exothermic or endothermic. Define enthalpy and explain its classification as a state function. \[δh_{solution} = δh_1 + δh_2 + δh_3.
from www.aiophotoz.com
Determine if a chemical process is exothermic or endothermic. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. By the end of this section, you will be able to: If the energy required to break apart the lattice is smaller than the. the enthalpy of solution can expressed as the sum of enthalpy changes for each step: Key takeaway enthalpy is a state function. \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. Use bond dissociation energies to calculate enthalpy change or heat of reaction. \[δh_{solution} = δh_1 + δh_2 + δh_3. the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure.
Enthalpy Diagram For Endothermic Reaction Diagram Media Images and
Endothermic Enthalpy Of Solution \[δh_{solution} = δh_1 + δh_2 + δh_3. Key takeaway enthalpy is a state function. the enthalpy of solution can expressed as the sum of enthalpy changes for each step: The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. If the energy required to break apart the lattice is smaller than the. Determine if a chemical process is exothermic or endothermic. State the first law of thermodynamics. Define enthalpy and explain its classification as a state function. if the energy required to break apart the lattice is greater than the energy released by hydration, enthalpy of solution is endothermic (+δh). Use bond dissociation energies to calculate enthalpy change or heat of reaction. the enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution. By the end of this section, you will be able to: \[δh_{solution} = δh_1 + δh_2 + δh_3.
From www.youtube.com
15.1 Enthalpy change of solution and hydration (HL) YouTube Endothermic Enthalpy Of Solution \[δh_{solution} = δh_1 + δh_2 + δh_3. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. Define enthalpy and explain its classification as a state function. \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. the enthalpy. Endothermic Enthalpy Of Solution.
From www.youtube.com
Change in enthalpy can be positive or negative Reactions meriSTEM Endothermic Enthalpy Of Solution \[δh_{solution} = δh_1 + δh_2 + δh_3. if the energy required to break apart the lattice is greater than the energy released by hydration, enthalpy of solution is endothermic (+δh). Use bond dissociation energies to calculate enthalpy change or heat of reaction. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic. Endothermic Enthalpy Of Solution.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Endothermic Enthalpy Of Solution Use bond dissociation energies to calculate enthalpy change or heat of reaction. the enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient. Endothermic Enthalpy Of Solution.
From thechemistrynotes.com
Enthalpy of solution and Hydration Endothermic Enthalpy Of Solution the enthalpy of solution can expressed as the sum of enthalpy changes for each step: \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. Determine if. Endothermic Enthalpy Of Solution.
From blogs.glowscotland.org.uk
Reaction Profiles & Enthalpy Change Higher Chemistry Unit 1 Endothermic Enthalpy Of Solution Key takeaway enthalpy is a state function. the enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution. Define enthalpy and explain its classification as a state function. \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. Use bond dissociation energies. Endothermic Enthalpy Of Solution.
From mudfooted.com
Endothermic Animals The Benefits Of Generating Your Own Heat MudFooted Endothermic Enthalpy Of Solution The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. if the energy required to break apart the lattice is greater than the energy released by hydration, enthalpy of solution is endothermic (+δh). the enthalpy of solution can expressed as. Endothermic Enthalpy Of Solution.
From www.slideserve.com
PPT Enthalpy Changes PowerPoint Presentation, free download ID2914427 Endothermic Enthalpy Of Solution The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. the enthalpy of solution can expressed as the sum of enthalpy changes for each step: if the energy required to break apart the lattice is greater than the energy released. Endothermic Enthalpy Of Solution.
From www.aiophotoz.com
Enthalpy Diagram For Endothermic Reaction Diagram Media Images and Endothermic Enthalpy Of Solution Key takeaway enthalpy is a state function. \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. if the energy required to break apart the lattice is greater than the energy released by hydration, enthalpy of solution is endothermic (+δh). Define enthalpy and explain its classification as a state function. \[δh_{solution} = δh_1 + δh_2 + δh_3.. Endothermic Enthalpy Of Solution.
From h-o-m-e.org
Chilling with Endothermic Reactions Endothermic Enthalpy Of Solution By the end of this section, you will be able to: the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. If the energy required to break apart the lattice is smaller than the. Determine if a chemical process is. Endothermic Enthalpy Of Solution.
From www.youtube.com
Enthalpies of Solution and Hydration Questions YouTube Endothermic Enthalpy Of Solution \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. Determine if a chemical process is exothermic or endothermic. Key takeaway enthalpy is a state function. State the first law of thermodynamics. the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of. Endothermic Enthalpy Of Solution.
From www.expii.com
Endothermic and Exothermic Reactions — Overview & Comparison Expii Endothermic Enthalpy Of Solution Use bond dissociation energies to calculate enthalpy change or heat of reaction. \[δh_{solution} = δh_1 + δh_2 + δh_3. if the energy required to break apart the lattice is greater than the energy released by hydration, enthalpy of solution is endothermic (+δh). If the energy required to break apart the lattice is smaller than the. Key takeaway enthalpy is. Endothermic Enthalpy Of Solution.
From jackwestin.com
Endothermic Exothermic Reactions Energy Changes In Chemical Reactions Endothermic Enthalpy Of Solution Define enthalpy and explain its classification as a state function. Key takeaway enthalpy is a state function. the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. the enthalpy of solution can expressed as the sum of enthalpy changes. Endothermic Enthalpy Of Solution.
From www.thesciencehive.co.uk
Lattice Enthalpy* — the science sauce Endothermic Enthalpy Of Solution the enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution. If the energy required to break apart the lattice is smaller than the. \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. the enthalpy of solution can expressed as. Endothermic Enthalpy Of Solution.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Enthalpy Of Solution If the energy required to break apart the lattice is smaller than the. \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. Use bond dissociation energies to calculate enthalpy. Endothermic Enthalpy Of Solution.
From chhattisgarh.pscnotes.com
Exothermic and endothermic reactions CGPCS Exam Preparation Endothermic Enthalpy Of Solution Determine if a chemical process is exothermic or endothermic. Define enthalpy and explain its classification as a state function. \[δh_{solution} = δh_1 + δh_2 + δh_3. By the end of this section, you will be able to: the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a. Endothermic Enthalpy Of Solution.
From 2012books.lardbucket.org
Enthalpy Endothermic Enthalpy Of Solution Key takeaway enthalpy is a state function. State the first law of thermodynamics. the enthalpy of solution can expressed as the sum of enthalpy changes for each step: Use bond dissociation energies to calculate enthalpy change or heat of reaction. the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a. Endothermic Enthalpy Of Solution.
From www.slideserve.com
PPT Measuring Enthalpy Changes PowerPoint Presentation, free download Endothermic Enthalpy Of Solution \[δh_{solution} = δh_1 + δh_2 + δh_3. By the end of this section, you will be able to: the enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution. if the energy required to break apart the lattice is greater than the energy. Endothermic Enthalpy Of Solution.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Enthalpy Of Solution Determine if a chemical process is exothermic or endothermic. if the energy required to break apart the lattice is greater than the energy released by hydration, enthalpy of solution is endothermic (+δh). the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent. Endothermic Enthalpy Of Solution.
From www.youtube.com
Enthalpy of Solution, Enthalpy of Hydration, Lattice Energy and Heat of Endothermic Enthalpy Of Solution \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. If the energy required to break apart the lattice is smaller than the. By the end of this section, you will be able to: State the first law of thermodynamics. the enthalpy of solution can expressed as the sum of enthalpy changes for each step: the. Endothermic Enthalpy Of Solution.
From general.chemistrysteps.com
What is Enthalpy Chemistry Steps Endothermic Enthalpy Of Solution \[δh_{solution} = δh_1 + δh_2 + δh_3. State the first law of thermodynamics. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. By the end of this section, you will be able to: the enthalpy of solution can expressed as. Endothermic Enthalpy Of Solution.
From schematicfixurner.z19.web.core.windows.net
Enthalpy Diagram Endothermic Endothermic Enthalpy Of Solution The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. the. Endothermic Enthalpy Of Solution.
From byjus.com
How electron gain enthalpy can be endothermic and exothermic? Endothermic Enthalpy Of Solution the enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. By the end of this. Endothermic Enthalpy Of Solution.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Endothermic Enthalpy Of Solution \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. If the energy required to break apart the lattice is smaller than the. the enthalpy of solution can expressed as the sum of enthalpy changes for each step: Key takeaway enthalpy is a state function. Use bond dissociation energies to calculate enthalpy change or heat of reaction.. Endothermic Enthalpy Of Solution.
From mavink.com
Endothermic Reaction Enthalpy Diagram Endothermic Enthalpy Of Solution The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. Define enthalpy and explain its classification as a state function. By the end of this section, you will be able to: If the energy required to break apart the lattice is smaller. Endothermic Enthalpy Of Solution.
From techiescientist.com
Is Condensation Endothermic or Exothermic? Techiescientist Endothermic Enthalpy Of Solution the enthalpy of solution can expressed as the sum of enthalpy changes for each step: If the energy required to break apart the lattice is smaller than the. Key takeaway enthalpy is a state function. By the end of this section, you will be able to: if the energy required to break apart the lattice is greater than. Endothermic Enthalpy Of Solution.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Enthalpy Of Solution the enthalpy of solution can expressed as the sum of enthalpy changes for each step: the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole. Endothermic Enthalpy Of Solution.
From www.nagwa.com
Question Video Classifying a Named Reaction as Endothermic or Endothermic Enthalpy Of Solution Determine if a chemical process is exothermic or endothermic. \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. State the first law of thermodynamics. If the energy. Endothermic Enthalpy Of Solution.
From www.science-revision.co.uk
Enthalpy changes in solution Endothermic Enthalpy Of Solution Use bond dissociation energies to calculate enthalpy change or heat of reaction. By the end of this section, you will be able to: the enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy. Endothermic Enthalpy Of Solution.
From www.thesciencehive.co.uk
Lattice Enthalpy* — the science sauce Endothermic Enthalpy Of Solution Use bond dissociation energies to calculate enthalpy change or heat of reaction. Key takeaway enthalpy is a state function. If the energy required to break apart the lattice is smaller than the. the enthalpy of solution can expressed as the sum of enthalpy changes for each step: Define enthalpy and explain its classification as a state function. if. Endothermic Enthalpy Of Solution.
From www.chemistrystudent.com
Enthalpy of Solution (Alevel) ChemistryStudent Endothermic Enthalpy Of Solution Key takeaway enthalpy is a state function. \[δh_{solution} = δh_1 + δh_2 + δh_3. the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. the enthalpy of. Endothermic Enthalpy Of Solution.
From www.chegg.com
Solved Calculate the enthalpies of solution for Li2SO4 and Endothermic Enthalpy Of Solution the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. Define enthalpy and explain its classification as a state function. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in. Endothermic Enthalpy Of Solution.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Endothermic Enthalpy Of Solution If the energy required to break apart the lattice is smaller than the. Define enthalpy and explain its classification as a state function. The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. the enthalpy of solution (δh soln) is the. Endothermic Enthalpy Of Solution.
From saylordotorg.github.io
Solutions Endothermic Enthalpy Of Solution the enthalpy of solution (δh soln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. the enthalpy of solution can expressed as the sum of enthalpy changes for each step: \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. The standard. Endothermic Enthalpy Of Solution.
From studylib.net
Enthalpy Change and Exothermic and Endothermic Reactions Endothermic Enthalpy Of Solution Determine if a chemical process is exothermic or endothermic. By the end of this section, you will be able to: The standard enthalpy change of solution (δhsolꝋ) is the enthalpy change when 1 mole of an ionic substance dissolves in sufficient water to form an infinitely dilute solution. If the energy required to break apart the lattice is smaller than. Endothermic Enthalpy Of Solution.
From www.slideserve.com
PPT Enthalpy Changes PowerPoint Presentation, free download ID2914427 Endothermic Enthalpy Of Solution \label{eq1}\] so the enthalpy of solution can either be endothermic, exothermic or. Define enthalpy and explain its classification as a state function. Determine if a chemical process is exothermic or endothermic. Key takeaway enthalpy is a state function. the enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give. Endothermic Enthalpy Of Solution.