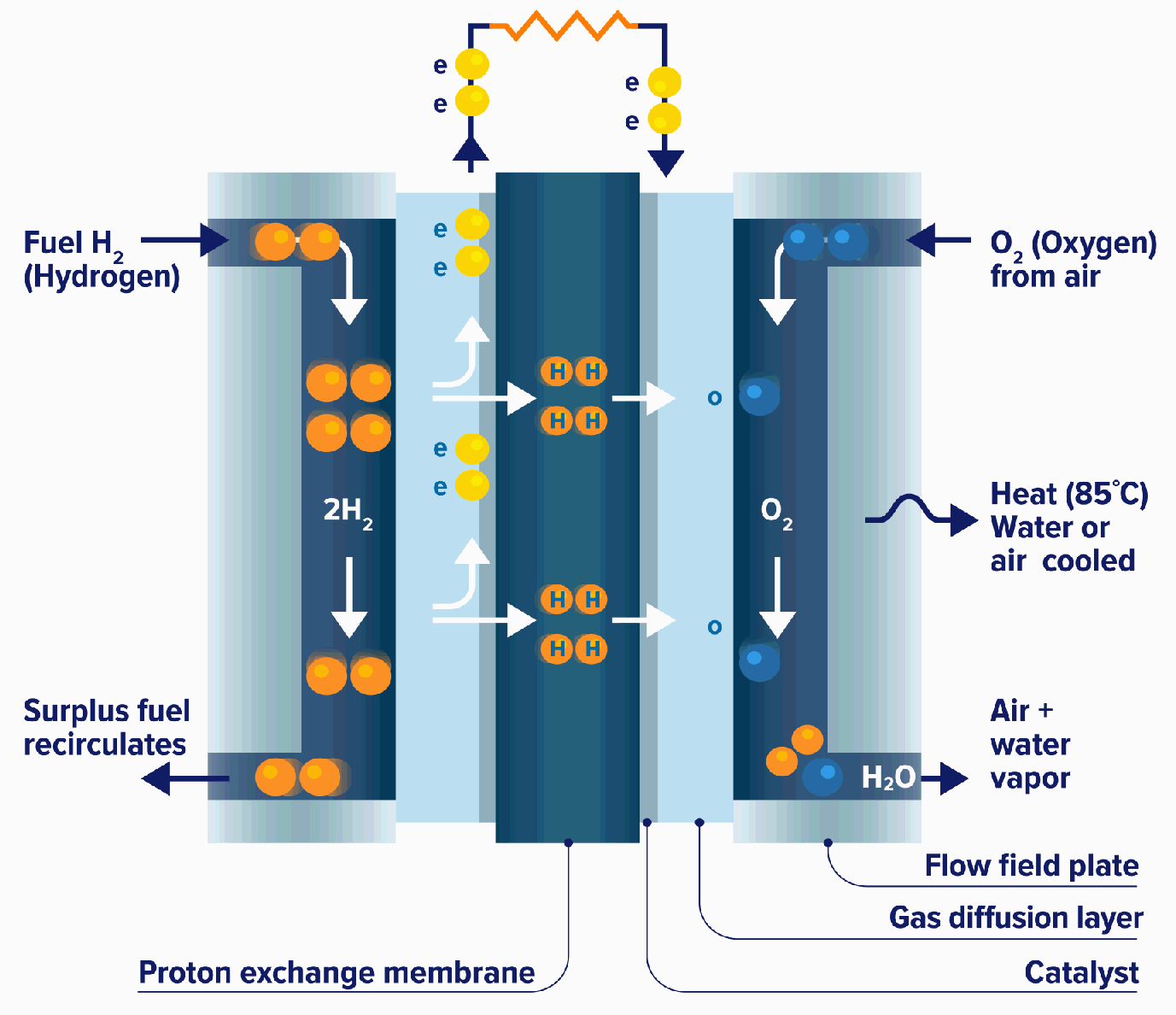
Fuel Cells Chemistry Igcse . (i) name the reactants in a fuel cell.[1] (ii) name the waste product of a fuel cell.[1] (d) electricity can be used to break down aqueous or molten ionic compounds. Which statements about a fuel cell are correct? a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. Hydrogen fuel cells generate electricity by combining hydrogen and oxygen in an electrochemical reaction, with the only byproduct being water. which gas is used as a fuel? fuel cells also convert chemical energy into electricity, but instead of having a fixed supply of chemicals inside the cell, they use external supplies of fuel and oxygen (or air). hydrogen fuel cells can be used to power cars. Hydrogen gas is used as fuel. Hydrogen is a commonly used fuel which. Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. A helium b hydrogen c nitrogen d oxygen. (c) fuel cells are used to generate electricity.
from www.fuelcellscars.com
Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. (i) name the reactants in a fuel cell.[1] (ii) name the waste product of a fuel cell.[1] (d) electricity can be used to break down aqueous or molten ionic compounds. hydrogen fuel cells can be used to power cars. oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. Hydrogen gas is used as fuel. A helium b hydrogen c nitrogen d oxygen. which gas is used as a fuel? Hydrogen fuel cells generate electricity by combining hydrogen and oxygen in an electrochemical reaction, with the only byproduct being water. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. (c) fuel cells are used to generate electricity.
ALL ABOUT FUEL CELLS HOW DO THEY WORK
Fuel Cells Chemistry Igcse which gas is used as a fuel? 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. A helium b hydrogen c nitrogen d oxygen. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Hydrogen fuel cells generate electricity by combining hydrogen and oxygen in an electrochemical reaction, with the only byproduct being water. Hydrogen is a commonly used fuel which. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. Which statements about a fuel cell are correct? (c) fuel cells are used to generate electricity. (i) name the reactants in a fuel cell.[1] (ii) name the waste product of a fuel cell.[1] (d) electricity can be used to break down aqueous or molten ionic compounds. hydrogen fuel cells can be used to power cars. which gas is used as a fuel? fuel cells also convert chemical energy into electricity, but instead of having a fixed supply of chemicals inside the cell, they use external supplies of fuel and oxygen (or air). oxidised (reacted with oxygen) within the fuel cell to produce a potential difference.
From www.youtube.com
GCSE Chemistry Hydrogen Fuel Cell Lesson YouTube Fuel Cells Chemistry Igcse A helium b hydrogen c nitrogen d oxygen. Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. Hydrogen is a commonly used fuel which. a fuel cell. Fuel Cells Chemistry Igcse.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Fuel Cells Chemistry Igcse Which statements about a fuel cell are correct? oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. Hydrogen is a commonly used fuel which. which gas is used as a fuel? Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. Hydrogen gas is used as fuel. (i) name the reactants. Fuel Cells Chemistry Igcse.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Fuel Cells Chemistry Igcse Hydrogen fuel cells generate electricity by combining hydrogen and oxygen in an electrochemical reaction, with the only byproduct being water. which gas is used as a fuel? hydrogen fuel cells can be used to power cars. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. Hydrogen gas is used as fuel. oxidised. Fuel Cells Chemistry Igcse.
From www.youtube.com
Fuel Cells Alevel Chemistry [ VIDEO UPDATED LINK IN DESCRIPTION👇 Fuel Cells Chemistry Igcse fuel cells also convert chemical energy into electricity, but instead of having a fixed supply of chemicals inside the cell, they use external supplies of fuel and oxygen (or air). 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. (c) fuel cells are used to generate electricity. hydrogen fuel. Fuel Cells Chemistry Igcse.
From www.savemyexams.com
Hydrogen Fuel Cells Cambridge O Level Chemistry Revision Notes 2023 Fuel Cells Chemistry Igcse a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. hydrogen fuel cells can be used to power cars. Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. fuel cells also convert chemical energy into electricity, but instead of. Fuel Cells Chemistry Igcse.
From www.thinkswap.com
Fuel Cells Chemistry Year 12 VCE Thinkswap Fuel Cells Chemistry Igcse oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. fuel cells also convert chemical energy into electricity, but instead of having a fixed. Fuel Cells Chemistry Igcse.
From www.researchgate.net
Scheme of a solid oxide fuel cell. Download Scientific Diagram Fuel Cells Chemistry Igcse Which statements about a fuel cell are correct? oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. (c) fuel cells are used to generate electricity. Hydrogen gas is used. Fuel Cells Chemistry Igcse.
From www.researchgate.net
Schematic representation of Direct Methanol Fuel Cell (DMFC). This fuel Fuel Cells Chemistry Igcse fuel cells also convert chemical energy into electricity, but instead of having a fixed supply of chemicals inside the cell, they use external supplies of fuel and oxygen (or air). (i) name the reactants in a fuel cell.[1] (ii) name the waste product of a fuel cell.[1] (d) electricity can be used to break down aqueous or molten ionic. Fuel Cells Chemistry Igcse.
From chem.libretexts.org
6.7 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cells Chemistry Igcse A helium b hydrogen c nitrogen d oxygen. 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. fuel cells also convert chemical energy into electricity, but instead of having a fixed supply of chemicals inside the cell, they use external supplies of fuel and oxygen (or air). which gas is. Fuel Cells Chemistry Igcse.
From www.youtube.com
GCSE Chemistry Revision "Fuel Cells" (Triple) YouTube Fuel Cells Chemistry Igcse (c) fuel cells are used to generate electricity. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. Hydrogen gas is used as fuel. which gas is used as a fuel? A helium b hydrogen c nitrogen. Fuel Cells Chemistry Igcse.
From www.youtube.com
GCSE Chemistry Fuel Cells 45 YouTube Fuel Cells Chemistry Igcse (i) name the reactants in a fuel cell.[1] (ii) name the waste product of a fuel cell.[1] (d) electricity can be used to break down aqueous or molten ionic compounds. oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. which. Fuel Cells Chemistry Igcse.
From www.youtube.com
Cells and Batteries for AQA 91 GCSE Chemistry and Trilogy Fuel Cells Chemistry Igcse One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. Which statements about a fuel cell are correct? Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. A helium b hydrogen c nitrogen d oxygen.. Fuel Cells Chemistry Igcse.
From www.youtube.com
Fuel Cells O Level IGCSE Chemistry YouTube Fuel Cells Chemistry Igcse Hydrogen fuel cells generate electricity by combining hydrogen and oxygen in an electrochemical reaction, with the only byproduct being water. A helium b hydrogen c nitrogen d oxygen. (i) name the reactants in a fuel cell.[1] (ii) name the waste product of a fuel cell.[1] (d) electricity can be used to break down aqueous or molten ionic compounds. 1 the. Fuel Cells Chemistry Igcse.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube Fuel Cells Chemistry Igcse A helium b hydrogen c nitrogen d oxygen. oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. Which statements about a fuel cell are correct? (i) name the reactants in a fuel cell.[1] (ii) name the waste product of a fuel cell.[1] (d) electricity can be used to break down aqueous or molten ionic compounds.. Fuel Cells Chemistry Igcse.
From users.highland.edu
Commercial Galvanic Cells Fuel Cells Chemistry Igcse (c) fuel cells are used to generate electricity. which gas is used as a fuel? Hydrogen is a commonly used fuel which. 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen. Fuel Cells Chemistry Igcse.
From www.youtube.com
Fuel Cell O level / IGCSE Chemistry YouTube Fuel Cells Chemistry Igcse a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. fuel cells also convert chemical energy into electricity, but instead of having a fixed supply of chemicals inside the cell, they. Fuel Cells Chemistry Igcse.
From www.reddit.com
[IGCSE Chem Fuel Cells, bonds] Q.b What are the different bonds Fuel Cells Chemistry Igcse 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. Which statements about a fuel cell are correct? Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. One primary application of hydrogen gas as a. Fuel Cells Chemistry Igcse.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind Fuel Cells Chemistry Igcse Which statements about a fuel cell are correct? which gas is used as a fuel? Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. Hydrogen is a. Fuel Cells Chemistry Igcse.
From revisechemistry.uk
Chemical Cells and Fuel Cells Edexcel T5 revisechemistry.uk Fuel Cells Chemistry Igcse which gas is used as a fuel? 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. Hydrogen fuel cells generate electricity by combining hydrogen and oxygen in an electrochemical reaction, with the only byproduct being water. fuel cells also convert chemical energy into electricity, but instead of having a fixed. Fuel Cells Chemistry Igcse.
From www.pinterest.co.uk
FREE GCSE Chemistry (Science) Hydrogen Fuel Cells Practice Exam Fuel Cells Chemistry Igcse hydrogen fuel cells can be used to power cars. which gas is used as a fuel? a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o.. Fuel Cells Chemistry Igcse.
From pressbooks.openedmb.ca
Batteries and Fuel Cells Chemistry and the Environment Fuel Cells Chemistry Igcse Which statements about a fuel cell are correct? 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. oxidised (reacted with oxygen) within the fuel cell to. Fuel Cells Chemistry Igcse.
From cmsw.mit.edu
» Small Steps and Giant Leaps in Fuel Cell Technologies Angles / 2022 Fuel Cells Chemistry Igcse which gas is used as a fuel? hydrogen fuel cells can be used to power cars. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. (c) fuel cells are used to generate electricity. Hydrogen fuel cells generate electricity by combining hydrogen. Fuel Cells Chemistry Igcse.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy Fuel Cells Chemistry Igcse (i) name the reactants in a fuel cell.[1] (ii) name the waste product of a fuel cell.[1] (d) electricity can be used to break down aqueous or molten ionic compounds. Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. which gas is used as a fuel? oxidised (reacted with oxygen) within the fuel cell. Fuel Cells Chemistry Igcse.
From chem.libretexts.org
8.5 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cells Chemistry Igcse (c) fuel cells are used to generate electricity. Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. Hydrogen gas is used as fuel. 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. hydrogen fuel cells can be used to power cars. a fuel cell is. Fuel Cells Chemistry Igcse.
From spsources.com
Why Solid Oxide Fuel Cells as a Remote Power Source Fuel Cells Chemistry Igcse hydrogen fuel cells can be used to power cars. (c) fuel cells are used to generate electricity. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. a fuel cell is an electrochemical cell in which a fuel donates electrons. Fuel Cells Chemistry Igcse.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Fuel Cells Chemistry Igcse a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. (i) name the reactants in a fuel cell.[1] (ii) name the waste product of a fuel cell.[1] (d) electricity can be used to break down aqueous or molten ionic compounds. a fuel cell is. Fuel Cells Chemistry Igcse.
From www.tes.com
Chemical Cells and Fuel Cells GCSE AQA Chemistry HIGHER Teaching Fuel Cells Chemistry Igcse a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A helium b hydrogen c nitrogen d oxygen. Which statements about a fuel cell are correct? Hydrogen fuel cells generate electricity by combining hydrogen and oxygen in an electrochemical reaction, with the only byproduct being. Fuel Cells Chemistry Igcse.
From chemistrycommunity.nature.com
Triple phase boundary in solid oxide fuel cells Reaction dynamics Fuel Cells Chemistry Igcse which gas is used as a fuel? Hydrogen gas is used as fuel. (c) fuel cells are used to generate electricity. Which statements about a fuel cell are correct? a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. hydrogen fuel cells. Fuel Cells Chemistry Igcse.
From www.youtube.com
Fuel Cell (0601) Thermodynamics Electrochem Reaction YouTube Fuel Cells Chemistry Igcse Hydrogen gas is used as fuel. (c) fuel cells are used to generate electricity. a fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. which gas is used as a fuel? A helium b hydrogen c nitrogen d oxygen. Overall reaction in a. Fuel Cells Chemistry Igcse.
From www.fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cells Chemistry Igcse oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. Which statements about a fuel cell are correct? (i) name the reactants in a fuel cell.[1] (ii) name the waste product of a fuel cell.[1] (d) electricity can be used to break. Fuel Cells Chemistry Igcse.
From iupac.org
Fuel Cells IUPAC International Union of Pure and Applied Chemistry Fuel Cells Chemistry Igcse 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. oxidised (reacted with oxygen) within the fuel cell to produce a potential difference. One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. (c) fuel cells are used to generate electricity. which gas is used as. Fuel Cells Chemistry Igcse.
From circuitlibackermann.z19.web.core.windows.net
A Fuel Cell Schematic Fuel Cells Chemistry Igcse Hydrogen is a commonly used fuel which. which gas is used as a fuel? Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. Hydrogen gas is used as fuel. Hydrogen fuel cells generate electricity by combining hydrogen and oxygen in an electrochemical reaction, with the only byproduct being water. oxidised (reacted with oxygen) within. Fuel Cells Chemistry Igcse.
From www.youtube.com
GCSE Chemistry Fuel Cells YouTube Fuel Cells Chemistry Igcse One primary application of hydrogen gas as a fuel is in hydrogen fuel cells. 1 the balanced equation for the reaction is h 2 + o 2 = h 2 o. Hydrogen is a commonly used fuel which. fuel cells also convert chemical energy into electricity, but instead of having a fixed supply of chemicals inside the cell, they. Fuel Cells Chemistry Igcse.
From www.youtube.com
OCR C6 Fuel Cells (Higher) YouTube Fuel Cells Chemistry Igcse A helium b hydrogen c nitrogen d oxygen. hydrogen fuel cells can be used to power cars. Which statements about a fuel cell are correct? Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. fuel cells also convert chemical energy into electricity, but instead of having a fixed supply of chemicals inside the cell,. Fuel Cells Chemistry Igcse.
From www.researchgate.net
6. Hydrogenoxygen fuel cell scheme. Download Scientific Diagram Fuel Cells Chemistry Igcse (c) fuel cells are used to generate electricity. Hydrogen fuel cells generate electricity by combining hydrogen and oxygen in an electrochemical reaction, with the only byproduct being water. Overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to. Hydrogen gas is used as fuel. One primary application of hydrogen gas as a fuel is in hydrogen. Fuel Cells Chemistry Igcse.