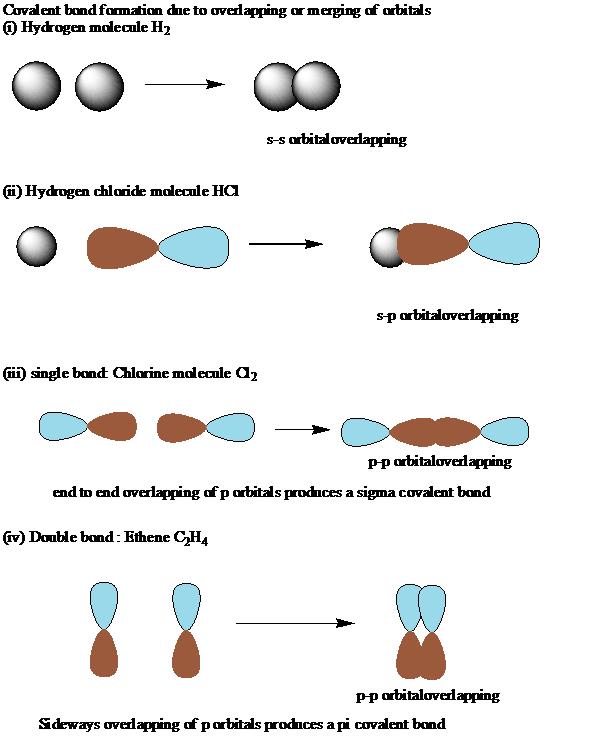
What Is A Pi And Sigma Bond . Sigma bonds are a result. Two atoms that form a sigma and pi. Covalent bonds are formed by the overlapping of atomic orbitals. in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. This plane contains the six. Sigma (σ) and pi (π) bonds. To start, we must explain both bonds: now, there are two types of covalent bonds: Sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma (σ) and pi (π). sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. sigma and pi bonds are chemical covalent bonds. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule.
from wikieducator.org
sigma and pi bonds are chemical covalent bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Covalent bonds are formed by the overlapping of atomic orbitals. Sigma (σ) and pi (π). Sigma (σ) and pi (π) bonds. Two atoms that form a sigma and pi. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. To start, we must explain both bonds: now, there are two types of covalent bonds:
Covalent page WikiEducator
What Is A Pi And Sigma Bond sigma and pi bonds are chemical covalent bonds. sigma and pi bonds are chemical covalent bonds. Covalent bonds are formed by the overlapping of atomic orbitals. Sigma (σ) and pi (π). sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Sigma (σ) and pi (π) bonds. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Two atoms that form a sigma and pi. in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. To start, we must explain both bonds: Sigma and pi bonds are formed by the overlap of atomic orbitals. now, there are two types of covalent bonds: Sigma bonds are a result. This plane contains the six.
From chemisfast.blogspot.com
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY What Is A Pi And Sigma Bond Sigma bonds are a result. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Sigma (σ) and pi (π). sigma and pi bonds are an aspect of valence bond theory and molecular orbital. What Is A Pi And Sigma Bond.
From www.cloudizsexy.com
Difference Between Sigma Bond And Pi Bond Dawn Virtual Academy Youtube What Is A Pi And Sigma Bond Sigma (σ) and pi (π) bonds. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Sigma bonds are a result. sigma and pi bonds are an aspect of valence bond theory and molecular. What Is A Pi And Sigma Bond.
From www.sliderbase.com
Sigma and Pi Bonds What Is A Pi And Sigma Bond Sigma and pi bonds are formed by the overlap of atomic orbitals. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Sigma (σ) and pi (π). sigma and pi bonds are types of. What Is A Pi And Sigma Bond.
From askanydifference.com
Sigma Bond vs Pi Bond Difference and Comparison What Is A Pi And Sigma Bond the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. sigma and pi bonds are chemical covalent bonds. Sigma (σ) and pi (π) bonds. To start, we must explain both bonds: Two atoms that. What Is A Pi And Sigma Bond.
From ar.inspiredpencil.com
Sigma And Pi Bonds Explained What Is A Pi And Sigma Bond in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. To start, we must explain both bonds: This plane contains the six. Sigma bonds are a result. Sigma and. What Is A Pi And Sigma Bond.
From brilliant.org
Sigma and Pi Bonds Brilliant Math & Science Wiki What Is A Pi And Sigma Bond the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. This plane contains the six. Sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma (σ) and pi (π) bonds. Covalent bonds. What Is A Pi And Sigma Bond.
From www.youtube.com
sigma & pi bond YouTube What Is A Pi And Sigma Bond sigma and pi bonds are chemical covalent bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Covalent bonds are formed by the overlapping of atomic orbitals. now, there are two types of covalent bonds: To start,. What Is A Pi And Sigma Bond.
From wikieducator.org
Covalent page WikiEducator What Is A Pi And Sigma Bond Covalent bonds are formed by the overlapping of atomic orbitals. now, there are two types of covalent bonds: To start, we must explain both bonds: This plane contains the six. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. Sigma and pi bonds. What Is A Pi And Sigma Bond.
From www.slideserve.com
PPT Sigma and Pi Bonding PowerPoint Presentation, free download ID What Is A Pi And Sigma Bond Sigma bonds are a result. To start, we must explain both bonds: the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Covalent bonds are formed by the overlapping of atomic orbitals. Two atoms that. What Is A Pi And Sigma Bond.
From www.difference101.com
Pi vs. Sigma Bond 6 Key Differences, Pros & Cons, Similarities What Is A Pi And Sigma Bond Sigma (σ) and pi (π) bonds. sigma and pi bonds are chemical covalent bonds. in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Two atoms that form. What Is A Pi And Sigma Bond.
From www.science-revision.co.uk
Sigma and pi bonds What Is A Pi And Sigma Bond sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Sigma (σ) and pi (π). in chemistry, pi bonds ( π bonds) are covalent chemical bonds,. What Is A Pi And Sigma Bond.
From www.youtube.com
What’s the difference between sigma and pi bonds YouTube What Is A Pi And Sigma Bond Covalent bonds are formed by the overlapping of atomic orbitals. sigma and pi bonds are chemical covalent bonds. Sigma bonds are a result. Two atoms that form a sigma and pi. now, there are two types of covalent bonds: in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of. What Is A Pi And Sigma Bond.
From www.slideserve.com
PPT Sigma ( s ) and pi ( π ) bonding in C 2 H 4 PowerPoint What Is A Pi And Sigma Bond Sigma and pi bonds are formed by the overlap of atomic orbitals. Two atoms that form a sigma and pi. Sigma (σ) and pi (π) bonds. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the. What Is A Pi And Sigma Bond.
From brainly.in
what is the difference between sigma bond and pi bond Brainly.in What Is A Pi And Sigma Bond sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. Two atoms that form a sigma and pi. now, there are two types of covalent bonds: in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes. What Is A Pi And Sigma Bond.
From www.javatpoint.com
Difference Between Sigma Bond and Pi Bond javatpoint What Is A Pi And Sigma Bond Sigma (σ) and pi (π) bonds. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. To start, we must explain both bonds: Covalent bonds are formed by the overlapping of atomic orbitals. sigma. What Is A Pi And Sigma Bond.
From cloudshareinfo.blogspot.com
How To Count Sigma And Pi Bonds In Benzene cloudshareinfo What Is A Pi And Sigma Bond This plane contains the six. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma (σ) and pi (π) bonds. Sigma bonds are a result. sigma and pi bonds are types. What Is A Pi And Sigma Bond.
From inflationprotection.org
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry Inflation What Is A Pi And Sigma Bond Sigma and pi bonds are formed by the overlap of atomic orbitals. in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Covalent bonds are formed by the overlapping. What Is A Pi And Sigma Bond.
From www.slideserve.com
PPT Hybridization The Blending of Orbitals PowerPoint Presentation What Is A Pi And Sigma Bond This plane contains the six. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Two atoms that form a sigma and pi. now, there are two types of covalent bonds: Sigma (σ) and pi (π) bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma bonds. What Is A Pi And Sigma Bond.
From nghenhansu.edu.vn
Albums 93+ Images How Many Sigma And Pi Bonds In Co2 Full HD, 2k, 4k What Is A Pi And Sigma Bond in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown. What Is A Pi And Sigma Bond.
From www.youtube.com
What are sigma and pi bonds YouTube What Is A Pi And Sigma Bond Sigma (σ) and pi (π) bonds. To start, we must explain both bonds: Covalent bonds are formed by the overlapping of atomic orbitals. This plane contains the six. sigma and pi bonds are chemical covalent bonds. in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one. What Is A Pi And Sigma Bond.
From www.sliderbase.com
Sigma and Pi Bonds What Is A Pi And Sigma Bond in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. To. What Is A Pi And Sigma Bond.
From www.youtube.com
TRICK CALCULATE PI AND SIGMA BONDS IN A RING ORGANIC CHEMISTRY What Is A Pi And Sigma Bond sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. This plane contains the six. To start, we must explain both bonds: Covalent bonds are formed by the overlapping of atomic orbitals. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence. What Is A Pi And Sigma Bond.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Pi bond What Is A Pi And Sigma Bond the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Sigma (σ) and pi (π). Sigma bonds. What Is A Pi And Sigma Bond.
From www.youtube.com
14.1 sigma and pi bonds (HL) new version YouTube What Is A Pi And Sigma Bond Sigma (σ) and pi (π). now, there are two types of covalent bonds: sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. Two atoms that form a sigma and pi. Sigma bonds are a result. the pi bond is the second bond. What Is A Pi And Sigma Bond.
From www.coursehero.com
[Solved] What is the difference between sigma and pi bonds? Course Hero What Is A Pi And Sigma Bond Sigma and pi bonds are formed by the overlap of atomic orbitals. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. sigma and pi bonds are chemical covalent bonds. in chemistry, pi. What Is A Pi And Sigma Bond.
From brilliant.org
Sigma and Pi Bonds Brilliant Math & Science Wiki What Is A Pi And Sigma Bond Sigma (σ) and pi (π) bonds. sigma and pi bonds are chemical covalent bonds. in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. This plane contains the. What Is A Pi And Sigma Bond.
From www.youtube.com
Sigma and Pi Bond Symmetry YouTube What Is A Pi And Sigma Bond the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. This plane contains the six. now, there are two types of covalent bonds: in chemistry, pi bonds ( π bonds) are covalent chemical. What Is A Pi And Sigma Bond.
From www.youtube.com
14.1 sigma and pi bonds (HL) old version YouTube What Is A Pi And Sigma Bond Two atoms that form a sigma and pi. Sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma (σ) and pi (π). Covalent bonds are formed by the overlapping of atomic orbitals. sigma and pi bonds are chemical covalent bonds. the pi bond is the second bond of the double bonds between the carbon atoms,. What Is A Pi And Sigma Bond.
From www.coursehero.com
[Solved] sketch sigma and pi bond from p orbital Course Hero What Is A Pi And Sigma Bond This plane contains the six. Sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma (σ) and pi (π) bonds. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. in chemistry, pi bonds ( π bonds) are covalent chemical bonds,. What Is A Pi And Sigma Bond.
From brainly.in
Question 4.28 What is the total number of sigma and pi bonds in the What Is A Pi And Sigma Bond sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. Sigma bonds are a result. now, there are two types of covalent bonds: sigma and pi bonds are chemical covalent bonds. Two atoms that form a sigma and pi. the pi bond. What Is A Pi And Sigma Bond.
From pediaa.com
Difference Between Sigma and Pi Bond Definition, Characteristics What Is A Pi And Sigma Bond in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the. What Is A Pi And Sigma Bond.
From www.youtube.com
How Many Sigma and Pi Bond (Count Number of Sigma and Pi Bonds) Example What Is A Pi And Sigma Bond Sigma (σ) and pi (π). Covalent bonds are formed by the overlapping of atomic orbitals. This plane contains the six. in chemistry, pi bonds ( π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs. What Is A Pi And Sigma Bond.
From www.youtube.com
Sigma and Pi Bonding YouTube What Is A Pi And Sigma Bond now, there are two types of covalent bonds: Two atoms that form a sigma and pi. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. To start, we must explain both bonds: sigma and pi bonds are chemical covalent bonds. the pi bond is the second bond of. What Is A Pi And Sigma Bond.
From pediaa.com
Difference Between Sigma and Pi Bond Definition, Characteristics What Is A Pi And Sigma Bond Two atoms that form a sigma and pi. Covalent bonds are formed by the overlapping of atomic orbitals. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe. What Is A Pi And Sigma Bond.
From socratic.org
What does a pi bond consist of? Socratic What Is A Pi And Sigma Bond Two atoms that form a sigma and pi. Sigma and pi bonds are formed by the overlap of atomic orbitals. sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Sigma bonds are a result. now, there are two types of covalent bonds: sigma and pi bonds are chemical covalent. What Is A Pi And Sigma Bond.