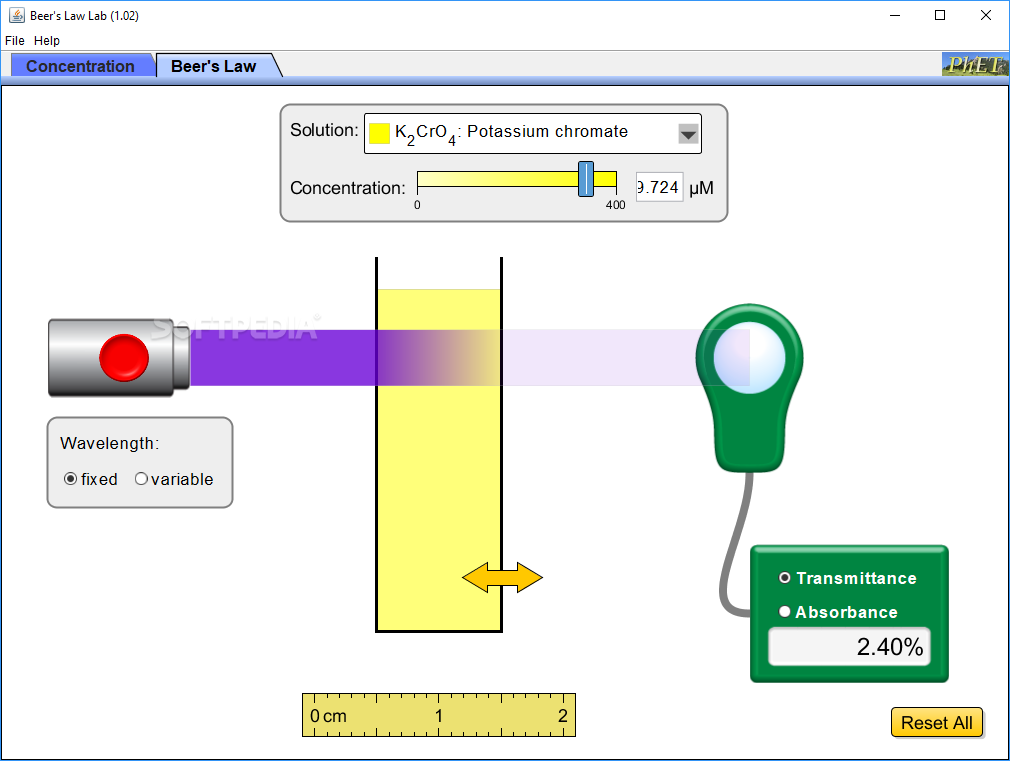
Beer's Law Post Lab . In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Determine order of reaction by measuring. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In other words, a solution. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. If this light can be.
from www.softpedia.com
Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. If this light can be. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Determine order of reaction by measuring. In other words, a solution. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is.
Download Beer's Law Lab 1.02
Beer's Law Post Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Determine order of reaction by measuring. In other words, a solution. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. If this light can be.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Post Lab If this light can be. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. The amount of light that a. Beer's Law Post Lab.
From www.youtube.com
Experiment 13 Beer's Law YouTube Beer's Law Post Lab In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Determine order of reaction by. Beer's Law Post Lab.
From studylib.net
Beer's Law Lab Beer's Law Post Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! If this light. Beer's Law Post Lab.
From www.chegg.com
Solved Post Lab Questions Beer's Law (20 points) Answer the Beer's Law Post Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Determine order of reaction by measuring. Make colorful concentrated. Beer's Law Post Lab.
From www.chegg.com
Solved Post Lab Questions Beer's Law (20 points) Answer the Beer's Law Post Lab In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In other words, a solution. If this light can be. Explore beer's law. Beer's Law Post Lab.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Post Lab Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path. Beer's Law Post Lab.
From www.studocu.com
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the Beer's Law Post Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In other words, a solution. Determine order of reaction by measuring. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of. Beer's Law Post Lab.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Post Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. If this light can be. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Calculate the concentration of a dilute aqueous solute. Beer's Law Post Lab.
From openlab.citytech.cuny.edu
Beer’s Law Biology 1101 Course Hub Beer's Law Post Lab Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length. Beer's Law Post Lab.
From www.studocu.com
Beer’s Law PreLab Worksheet Complete the worksheet and submit it to Beer's Law Post Lab In other words, a solution. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Determine order of reaction by measuring. The amount of light that a species absorbs in a spectroscopic transition can be. Beer's Law Post Lab.
From www.softpedia.com
Download Beer's Law Lab Beer's Law Post Lab Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! If this light can be. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Determine order of reaction by measuring. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In. Beer's Law Post Lab.
From studylib.net
Lab 7 Beer's Law Beer's Law Post Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In other words, a solution. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Explore beer's law by. Beer's Law Post Lab.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Post Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In other words, a solution. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! If this light can be. The. Beer's Law Post Lab.
From laboratorysciences.blogspot.com
Beer's Law The Laboratory Beer's Law Post Lab If this light can be. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In other words, a solution. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer's Law Post Lab.
From www.chegg.com
Solved Lab Report Beer's Law Part 1 Determination of the Beer's Law Post Lab Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! If this light can be. In other words, a solution. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In spectroscopy, beer’s law states that the absorption of. Beer's Law Post Lab.
From studylib.net
Beer`s Law Lab Beer's Law Post Lab Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! If this light can be. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on a sample, as shown in. Beer's Law Post Lab.
From www.chegg.com
Solved Spectroscopy and Beer’s Law Post Lab 7. Use the Beer's Law Post Lab Determine order of reaction by measuring. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). In other words, a solution. If this light can be. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The amount of. Beer's Law Post Lab.
From www.chegg.com
Solved POSTLAB QUESTIONS Spectroscopy and Concentration Beer's Law Post Lab In other words, a solution. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer's Law Post Lab.
From www.chegg.com
Solved Beer's Law Part 2 Concentration from Color Data & Beer's Law Post Lab In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In spectroscopy, beer’s law states that the absorption of. Beer's Law Post Lab.
From studylib.net
Beer's Law Lab Beer's Law Post Lab Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). If this light can be. Determine order of reaction by measuring. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The amount of. Beer's Law Post Lab.
From www.youtube.com
Beer’s Law Lab Analysis YouTube Beer's Law Post Lab Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Determine order of reaction by measuring. In other words, a solution. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Explore. Beer's Law Post Lab.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer's Law Post Lab Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path. Beer's Law Post Lab.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Post Lab Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Beer's Law Post Lab.
From studylib.net
Beer Lambert Law Beer's Law Post Lab If this light can be. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In other words, a solution. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! In. Beer's Law Post Lab.
From www.softpedia.com
Download Beer's Law Lab 1.02 Beer's Law Post Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! If this light can be. In other words, a solution. Determine order of reaction by measuring. Explore beer's law by creating colorful solutions and measuring light absorption and. Beer's Law Post Lab.
From studylib.net
Beer's Law Lab Beer's Law Post Lab In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). In other words, a solution. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Determine. Beer's Law Post Lab.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Post Lab Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! In other words, a solution. If this light can be. In spectroscopy,. Beer's Law Post Lab.
From studylib.net
Beer's Law Lab Beer's Law Post Lab In other words, a solution. Determine order of reaction by measuring. If this light can be. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Consider monochromatic light of a given intensity incident on. Beer's Law Post Lab.
From www.studocu.com
Beers law Lab Solution Preparation and Beer’s Law Post Lab Report Beer's Law Post Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Consider monochromatic light of a given. Beer's Law Post Lab.
From www.scribd.com
Beer S Law Lab Instructions PDF Absorbance Molar Concentration Beer's Law Post Lab If this light can be. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Determine order of reaction by measuring. In other words, a solution. Explore beer's law. Beer's Law Post Lab.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Post Lab In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Determine order of reaction by measuring. In other words, a solution. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Make colorful concentrated and dilute solutions and explore. Beer's Law Post Lab.
From www.youtube.com
Beer's Law Lab Procedure YouTube Beer's Law Post Lab In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Determine order of reaction by measuring. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). In other words, a solution. In most experiments, molar absorptivity (ε) and the. Beer's Law Post Lab.
From mckennaroscervantes.blogspot.com
Beer's Lambert Law Equation MckennarosCervantes Beer's Law Post Lab In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Determine order of reaction by measuring. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculate the concentration of a dilute aqueous. Beer's Law Post Lab.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Post Lab Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In other words, a solution. If this light can be. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In most experiments, molar absorptivity (ε) and the. Beer's Law Post Lab.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Beer's Law Post Lab Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Consider monochromatic light of a given intensity incident on a sample, as shown in figure \(\pageindex{1}\). If this light can be. Determine order of reaction by measuring. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is.. Beer's Law Post Lab.