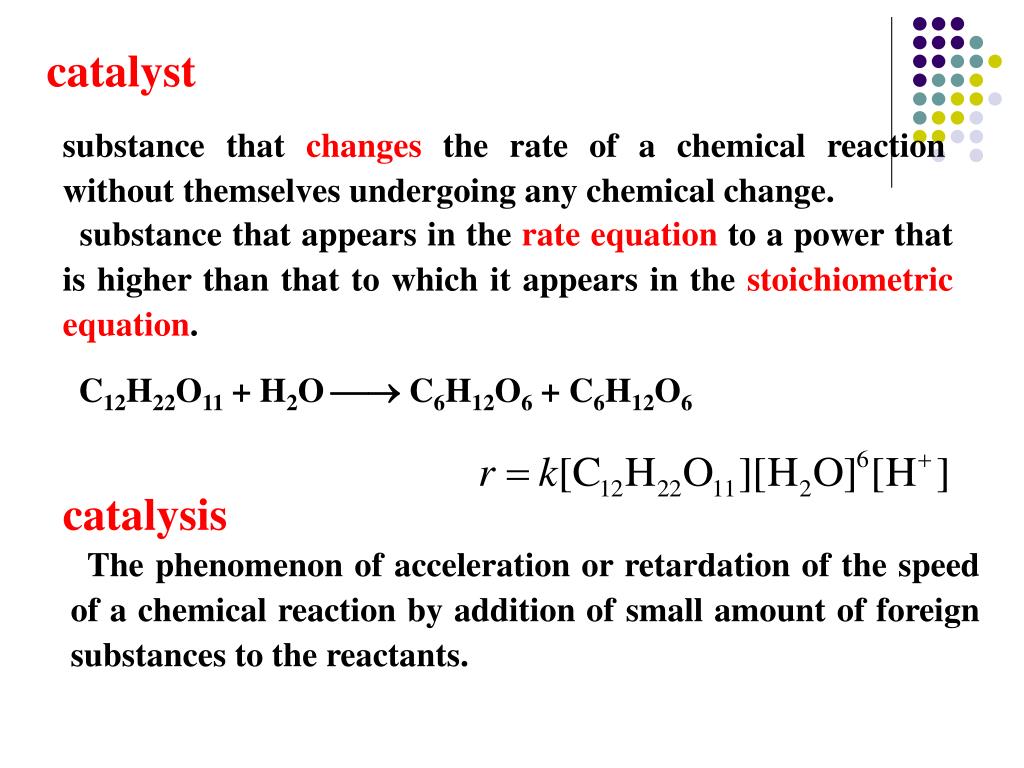
Catalyst In A Chemical Reaction Activated Complex . explain how an activated complex differs from an intermediate. Define catalyst, and sketch out an activation. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. The activation energy of a chemical. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. reaction diagrams for a chemical process with and without a catalyst are shown below. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. an activated complex is the structure that results in the maximum energy point along the reaction path.
from exotukoyg.blob.core.windows.net
higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. The activation energy of a chemical. Define catalyst, and sketch out an activation. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. reaction diagrams for a chemical process with and without a catalyst are shown below. explain how an activated complex differs from an intermediate. an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. an activated complex is the structure that results in the maximum energy point along the reaction path. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy.
Catalyst Reaction Chemical Equation at Glenn Leopard blog
Catalyst In A Chemical Reaction Activated Complex catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Define catalyst, and sketch out an activation. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. explain how an activated complex differs from an intermediate. an activated complex is the structure that results in the maximum energy point along the reaction path. reaction diagrams for a chemical process with and without a catalyst are shown below. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). The activation energy of a chemical.
From 2012books.lardbucket.org
Catalysis Catalyst In A Chemical Reaction Activated Complex Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. catalysts affect the rate. Catalyst In A Chemical Reaction Activated Complex.
From exotukoyg.blob.core.windows.net
Catalyst Reaction Chemical Equation at Glenn Leopard blog Catalyst In A Chemical Reaction Activated Complex Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). explain how an activated complex differs from an intermediate. an activated complex is the structure that results in the maximum energy point along the reaction path. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide. Catalyst In A Chemical Reaction Activated Complex.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst In A Chemical Reaction Activated Complex explain how an activated complex differs from an intermediate. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. The activation energy of a chemical. an activated complex is the structure that results in the maximum energy point along the reaction path. catalysts affect the rate of a chemical reaction. Catalyst In A Chemical Reaction Activated Complex.
From www.slideserve.com
PPT Role of Catalysts PowerPoint Presentation, free download ID2823169 Catalyst In A Chemical Reaction Activated Complex Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. an activated complex is. Catalyst In A Chemical Reaction Activated Complex.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst In A Chemical Reaction Activated Complex catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). higher temperatures can provide enough energy. Catalyst In A Chemical Reaction Activated Complex.
From www.pinterest.com
Catalyst Easy Science Energy activities, Chemical reactions Catalyst In A Chemical Reaction Activated Complex this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. Define catalyst, and sketch out an activation. an activated complex is the structure that results in the maximum energy point along the reaction path. The activation energy of a chemical. explain how an activated complex differs from an intermediate. Catalysts can. Catalyst In A Chemical Reaction Activated Complex.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst In A Chemical Reaction Activated Complex Define catalyst, and sketch out an activation. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. reaction diagrams for a chemical process with and without a catalyst are shown below. Catalysts can. Catalyst In A Chemical Reaction Activated Complex.
From www.purechemistry.org
Activated complex theory (ACT) of reaction rate Purechemistry Catalyst In A Chemical Reaction Activated Complex The activation energy of a chemical. explain how an activated complex differs from an intermediate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). an activated complex is the. Catalyst In A Chemical Reaction Activated Complex.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube Catalyst In A Chemical Reaction Activated Complex higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. an activated complex is an unstable. Catalyst In A Chemical Reaction Activated Complex.
From exorbcrky.blob.core.windows.net
Catalyst Meaning Chem at Rick Franks blog Catalyst In A Chemical Reaction Activated Complex Define catalyst, and sketch out an activation. The activation energy of a chemical. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). an activated complex is the structure that results in the maximum energy point along the reaction path. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h]. Catalyst In A Chemical Reaction Activated Complex.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst In A Chemical Reaction Activated Complex catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. Define catalyst, and sketch out an activation. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than. Catalyst In A Chemical Reaction Activated Complex.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst In A Chemical Reaction Activated Complex an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. The activation energy of a chemical. an activated complex is the structure that results in the maximum energy point along the reaction path.. Catalyst In A Chemical Reaction Activated Complex.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst In A Chemical Reaction Activated Complex this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. reaction diagrams for a chemical process with and without a catalyst are shown below. The activation energy of a chemical. an activated complex is the structure that results in the maximum energy point along the reaction path. Catalysts can be homogenous. Catalyst In A Chemical Reaction Activated Complex.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst In A Chemical Reaction Activated Complex Define catalyst, and sketch out an activation. an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing.. Catalyst In A Chemical Reaction Activated Complex.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst In A Chemical Reaction Activated Complex catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. an activated complex is the structure that results in the maximum energy point along the reaction path. Define catalyst, and sketch out an. Catalyst In A Chemical Reaction Activated Complex.
From dxoxxqpqm.blob.core.windows.net
Catalytic Molecule Meaning at Shirley Hall blog Catalyst In A Chemical Reaction Activated Complex Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. an activated complex is the structure that results in the maximum energy point along the reaction path. Define catalyst, and sketch out an. Catalyst In A Chemical Reaction Activated Complex.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst In A Chemical Reaction Activated Complex explain how an activated complex differs from an intermediate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The activation energy of a chemical. Define catalyst, and sketch out an activation. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the. Catalyst In A Chemical Reaction Activated Complex.
From www.pinterest.com
Activated Complex Easy Science Flashcards, Easy science, Energy Catalyst In A Chemical Reaction Activated Complex an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. an activated complex is the structure that results in the maximum energy point along the reaction path. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. explain how an. Catalyst In A Chemical Reaction Activated Complex.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst In A Chemical Reaction Activated Complex higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. an activated complex is the structure that results in the maximum energy point along the reaction path. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). explain how an activated complex. Catalyst In A Chemical Reaction Activated Complex.
From exoluszth.blob.core.windows.net
Catalyst Examples Pictures at Mandy Simpkins blog Catalyst In A Chemical Reaction Activated Complex Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below. Define catalyst, and sketch out an activation.. Catalyst In A Chemical Reaction Activated Complex.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalyst In A Chemical Reaction Activated Complex this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). an activated complex is. Catalyst In A Chemical Reaction Activated Complex.
From www.slideserve.com
PPT Rates of Reaction & Equilibrium PowerPoint Presentation, free Catalyst In A Chemical Reaction Activated Complex explain how an activated complex differs from an intermediate. The activation energy of a chemical. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Define catalyst, and sketch out an activation. an activated complex is the structure that results in the maximum energy point along the reaction path.. Catalyst In A Chemical Reaction Activated Complex.
From www.slideserve.com
PPT Activated complex theory PowerPoint Presentation ID3995760 Catalyst In A Chemical Reaction Activated Complex Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). reaction diagrams for a chemical process with and without a catalyst are shown below. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. catalysts affect the rate of a chemical reaction. Catalyst In A Chemical Reaction Activated Complex.
From socratic.org
What is an activated complex? Socratic Catalyst In A Chemical Reaction Activated Complex an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. explain how an activated complex differs from an intermediate. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a. Catalyst In A Chemical Reaction Activated Complex.
From chemistnotes.com
What is Activated complex? Easy Explanation Chemistry Notes Catalyst In A Chemical Reaction Activated Complex an activated complex is the structure that results in the maximum energy point along the reaction path. explain how an activated complex differs from an intermediate. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different. Catalyst In A Chemical Reaction Activated Complex.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst In A Chemical Reaction Activated Complex explain how an activated complex differs from an intermediate. reaction diagrams for a chemical process with and without a catalyst are shown below. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The activation energy of a chemical. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h]. Catalyst In A Chemical Reaction Activated Complex.
From www.cheric.org
Chemical Reaction (Reaction rate) Catalyst In A Chemical Reaction Activated Complex The activation energy of a chemical. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). Define catalyst, and sketch out an activation. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. an activated complex is the structure that results in the. Catalyst In A Chemical Reaction Activated Complex.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst In A Chemical Reaction Activated Complex Define catalyst, and sketch out an activation. higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. an activated complex is the structure that results in the maximum energy point along the reaction path. reaction diagrams for a chemical process with and without a catalyst are shown below. explain how. Catalyst In A Chemical Reaction Activated Complex.
From www.chm.uri.edu
CHM 112 Lecture 6 Catalyst In A Chemical Reaction Activated Complex Define catalyst, and sketch out an activation. The activation energy of a chemical. reaction diagrams for a chemical process with and without a catalyst are shown below. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide. Catalyst In A Chemical Reaction Activated Complex.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst In A Chemical Reaction Activated Complex Define catalyst, and sketch out an activation. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). an activated complex is the structure that results in the maximum energy point along the reaction. Catalyst In A Chemical Reaction Activated Complex.
From www.slideshare.net
Biology 2.4 Catalyst In A Chemical Reaction Activated Complex explain how an activated complex differs from an intermediate. Define catalyst, and sketch out an activation. an activated complex is the structure that results in the maximum energy point along the reaction path. reaction diagrams for a chemical process with and without a catalyst are shown below. The activation energy of a chemical. an activated complex. Catalyst In A Chemical Reaction Activated Complex.
From www.waca.msf.org
Catalyst, Catalyst Catalyst In A Chemical Reaction Activated Complex higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. Define catalyst, and sketch out an activation. reaction diagrams for a chemical process with and without a catalyst are shown below. this reaction involves the oxidation of tartaric acid [ho2cch(oh)ch(oh)co2h] by hydrogen peroxide in the presence of. Catalysts can be homogenous. Catalyst In A Chemical Reaction Activated Complex.
From www.youtube.com
Catalysts Chemistry Khan Academy YouTube Catalyst In A Chemical Reaction Activated Complex an activated complex is the structure that results in the maximum energy point along the reaction path. Define catalyst, and sketch out an activation. explain how an activated complex differs from an intermediate. reaction diagrams for a chemical process with and without a catalyst are shown below. catalysts affect the rate of a chemical reaction by. Catalyst In A Chemical Reaction Activated Complex.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst In A Chemical Reaction Activated Complex explain how an activated complex differs from an intermediate. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants). an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. Define catalyst, and sketch out an activation. higher temperatures can provide. Catalyst In A Chemical Reaction Activated Complex.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst In A Chemical Reaction Activated Complex higher temperatures can provide enough energy to help more molecules reach the activated complex, thus increasing. an activated complex is an unstable arrangement of atoms that exists momentarily at the peak of the activation. The activation energy of a chemical. explain how an activated complex differs from an intermediate. an activated complex is the structure that. Catalyst In A Chemical Reaction Activated Complex.