How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine . Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). Potassium iodate is used as a titrant and is added to an. Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Add 2 ml of the sulfuric acid mixture and about 25 ml of.
from www.numerade.com
Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. Potassium iodate is used as a titrant and is added to an. Add 2 ml of the sulfuric acid mixture and about 25 ml of. Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of.
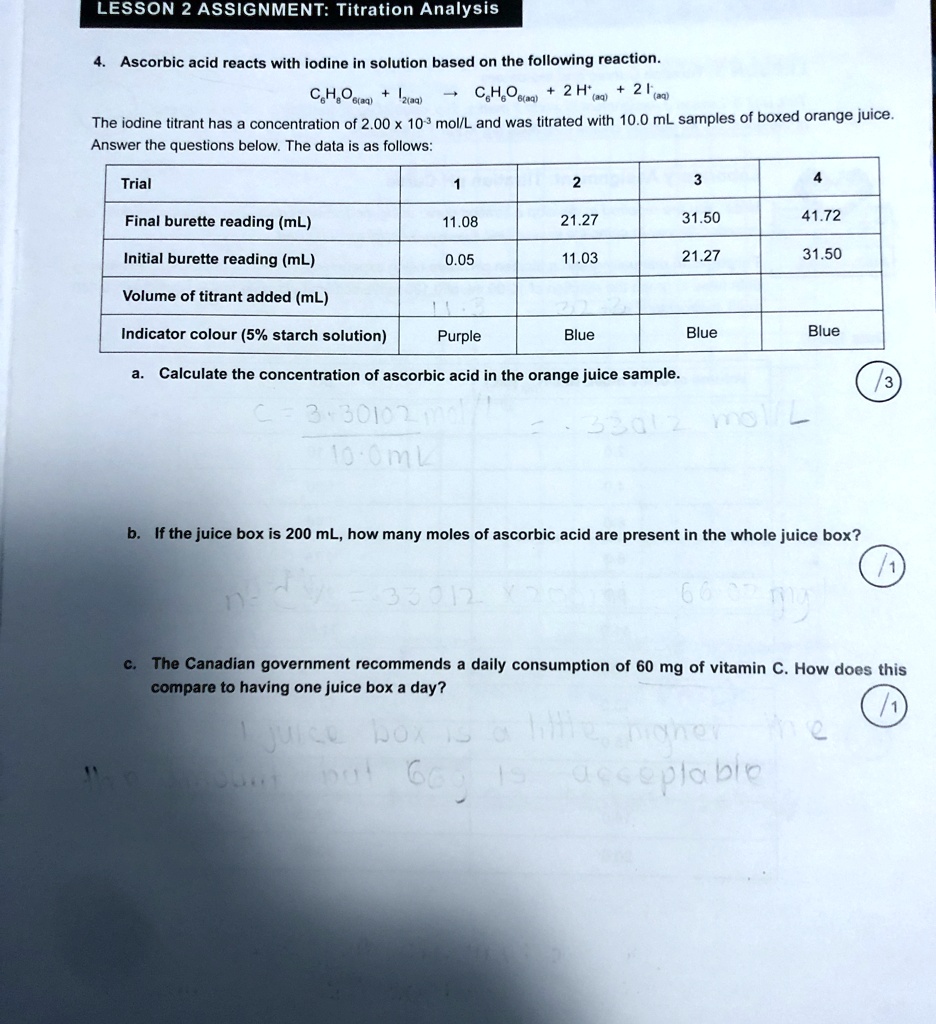
SOLVED Analytical Chem 20 LESSON 2 ASSIGNMENT Titration Analysis
How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. Potassium iodate is used as a titrant and is added to an. This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). Add 2 ml of the sulfuric acid mixture and about 25 ml of. Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c.
From studylib.net
TITRATION OF VITAMIN C How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Add 2 ml of the sulfuric acid mixture and about 25 ml of. Potassium iodate is used as a titrant and is added to an. In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. Oxidized in the reaction i 2 (the oxidizing agent) is generated by. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From studylib.net
Determination of Vitamin C Concentration by Titration How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. Add 2 ml of the sulfuric acid mixture and about 25 ml of. Potassium iodate is used as a titrant and is added to an. In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From childhealthpolicy.vumc.org
Vitamin c determination by iodine titration lab report. Vitamin C How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. Potassium iodate is used as a titrant and is added to an. Oxidized in. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.scribd.com
Ascorbic Acid Iodometric Titration Titration Iodine How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). Add 2 ml of the sulfuric acid mixture and about 25 ml of. Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.chegg.com
Solved Calculate the concentration of ascorbic acid in each How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. Add 2 ml of the sulfuric acid mixture and about 25 ml of. Oxidized. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.slideshare.net
Vitamin c titration experiment How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. Add 2 ml of the sulfuric acid mixture and about 25 ml of. Be sure to record the concentration of the. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.compoundchem.com
A Guide to Acids, Acid Strength, and Concentration Compound Interest How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Add 2 ml of the sulfuric acid mixture and about 25 ml of. In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. Potassium iodate is used as a titrant and is added. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.chegg.com
Solved LABORATORY 10 DETERMINATION OF VITAMIN C How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction.. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From lulelaboratory.blogspot.com
Lu Le Laboratory The Determination of Ascorbic Acid in Vitamin C How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Add 2 ml of the sulfuric acid mixture and about 25 ml of. Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From studylib.net
Vitamin C DCPIP titration How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. Add 2 ml of the sulfuric acid mixture and about 25 ml of. This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Potassium iodate is. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.youtube.com
Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) to be dissolved How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). Potassium iodate is used as a titrant and is added to an. Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. A suitable method for the determination of vitamin c (c 6. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.youtube.com
NCERT Solution Intext 2.11 Calculate the mass of ascorbic acid How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From theedge.com.hk
Chemistry How To Titration The Edge How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.chegg.com
Solved LABORATORY 10 DETERMINATION OF VITAMIN C How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. A suitable method for the determination of vitamin c (c 6 h 8 o. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.numerade.com
SOLVED 72 Titration Calculations Ascorbic acid (vitamin C) reacts How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Potassium iodate is used as a titrant and is added to an. A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution.. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.slideshare.net
Vitamin c powerpoint How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From vitaminwalls.blogspot.com
Determination Of Vitamin C By Redox Titration With Iodate How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Potassium iodate is used as a titrant and is added to an. A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.studocu.com
Lab 4 Iodometric Titration of Vitamin C Lab 4 Ascorbic Acid How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. Add 2 ml of the sulfuric acid mixture and about 25 ml of. This method determines the vitamin c concentration in a solution. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.numerade.com
SOLVED Analytical Chem 20 LESSON 2 ASSIGNMENT Titration Analysis How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Add 2 ml of the sulfuric acid mixture and about 25 ml of. Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. A suitable method. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From keplarllp.com
😎 Vitamin c determination by iodine titration lab report. Vitamin C How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Potassium iodate is used as a titrant and is added to an. A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). Oxidized in. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From slideplayer.com
Determination of Vitamin C Concentration by Titration ppt video How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Potassium iodate is used as a titrant and is added to an. Add 2 ml of the sulfuric acid mixture and about 25 ml of.. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From childhealthpolicy.vumc.org
Vitamin c determination by iodine titration lab report. Vitamin C How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). Oxidized in the reaction i 2 (the oxidizing agent) is generated by. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.numerade.com
SOLVED Vitamin C (ascorbic acid, C6H8O6, 176.12 g / mol ) can be How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). A suitable method for the determination of vitamin c (c 6 h. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.numerade.com
SOLVED Determination of Vitamin C by Titration Objectives Data and How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.vrogue.co
25 Principle Of Vitamin C Determination By Visual Tit vrogue.co How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. This method determines the vitamin c concentration in a solution by a. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.numerade.com
SOLVED Vitamin C (ascorbic acid, C6H8O6, 176.12 g / mol ) can be How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. In the first part, you will prepare a primary standard. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From slideplayer.com
Determination of Vitamin C Concentration by Titration ppt video How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). Add 2 ml of the sulfuric acid mixture and about 25 ml of. This. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From studylib.net
3Determination of Ascorbic Acid in Vitamin C How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Potassium iodate is used as a titrant and is added to an. This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. Calculate milligrams of ascorbic acid per gram of sample and using the average. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.coursehero.com
Vitamin C Titration Part I. Using the mean volume of your two (or How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. Add 2 ml of the sulfuric acid mixture and about 25 ml of. Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. This method determines the vitamin c concentration in. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From biochemistrygirls.blogspot.com
BIOCHEMISTRY Experiment 4 Vitamin C How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). Potassium iodate is used as a titrant and is added to an. In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. A suitable. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.chegg.com
Solved The balanced equation for the reaction between iodine How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. Calculate milligrams of ascorbic acid per gram of sample and using the average mass. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.slideshare.net
analysis vitamin c in commercial fruit juice How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine In the first part, you will prepare a primary standard potassium iodate solution (kio3) and use it to standardize a sodium thiosulfate solution. This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From www.youtube.com
Determination of Vitamin C in Tablet by using Iodine Solution Fruit How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine A suitable method for the determination of vitamin c (c 6 h 8 o 6 ) is a titration with potassium iodate (kio 3 ). Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). Oxidized in the reaction i 2 (the oxidizing agent) is generated by. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From pharmacypdfnotes.blogspot.com
Estimation of Vitamin C Concentration By Titration Installation (Redox How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). Calculate milligrams of ascorbic acid per gram of sample and using the average mass of a tablet, determine the number of milligrams of vitamin c. Potassium iodate is used as a titrant and is added to an.. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.
From webmis.highland.cc.il.us
Quantitative Analysis Using Titrations How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine Be sure to record the concentration of the standard ascorbic acid (it should be about 0.500 g of vitamin c per 1.000 l). Oxidized in the reaction i 2 (the oxidizing agent) is generated by the reaction. This method determines the vitamin c concentration in a solution by a redox titration with potassium iodate in the presence of. Add 2. How To Calculate Concentration Of Vitamin C In Titration Of Ascorbic Acid With Iodine.