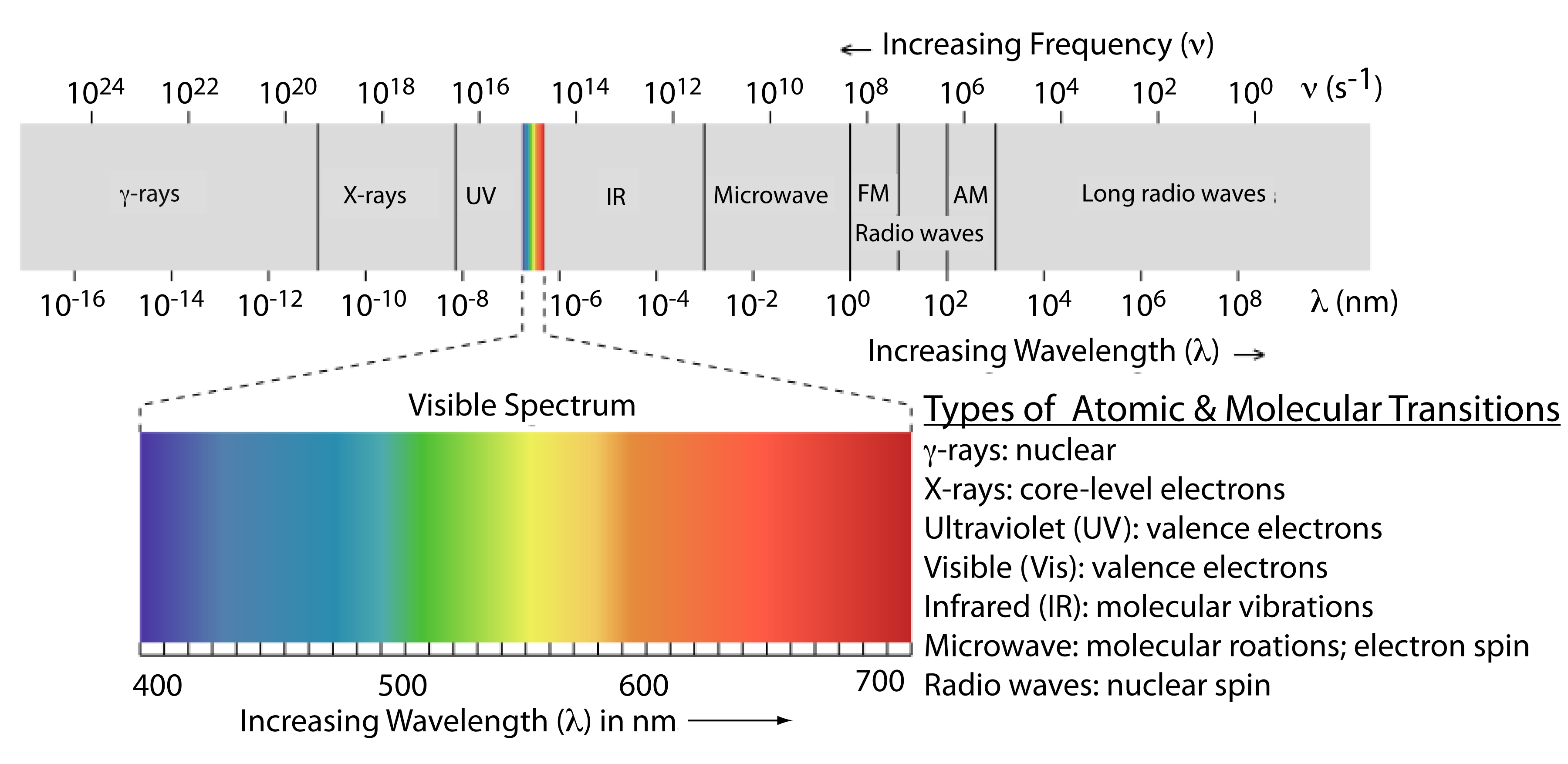
Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy . The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. What is actually being observed spectroscopically is. 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. 1.2 wavelength and frequency 3.
from chem.libretexts.org
What is actually being observed spectroscopically is. 1.2 wavelength and frequency 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. To understand why some compounds are colored and others are not, and to determine the relationship. 1.1 the electromagnetic spectrum 3.
10.1 Overview of Spectroscopy Chemistry LibreTexts
Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. What is actually being observed spectroscopically is. To understand why some compounds are colored and others are not, and to determine the relationship. 1.2 wavelength and frequency 3. 1.1 the electromagnetic spectrum 3.
From www.slideshare.net
UVvisible Spectroscopy Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy What is actually being observed spectroscopically is. 1.2 wavelength and frequency 3. 1.1 the electromagnetic spectrum 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. To understand why some compounds are colored and others are not, and to determine the relationship. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT Electronic (UVvisible) Spectroscopy PowerPoint Presentation Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.2 wavelength and frequency 3. What is actually being observed spectroscopically is. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT UltravioletVisible Spectroscopy PowerPoint Presentation, free Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.2 wavelength and frequency 3. 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. What is actually being observed spectroscopically is. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy To understand why some compounds are colored and others are not, and to determine the relationship. 1.2 wavelength and frequency 3. What is actually being observed spectroscopically is. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.1 the electromagnetic spectrum 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT Lecture 36 Electronic spectroscopy PowerPoint Presentation, free Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.2 wavelength and frequency 3. What is actually being observed spectroscopically is. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideshare.net
Uv visible spectroscopy ppt Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.2 wavelength and frequency 3. 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. What is actually being observed spectroscopically is. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.studypool.com
SOLUTION Uv visible spectroscopy electronic Studypool Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy What is actually being observed spectroscopically is. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. To understand why some compounds are colored and others are not, and to determine the relationship. 1.2 wavelength and frequency 3. 1.1 the electromagnetic spectrum 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT Optical Electronic Spectroscopy 1 PowerPoint Presentation, free Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy What is actually being observed spectroscopically is. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. 1.2 wavelength and frequency 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.researchgate.net
(PDF) UVVisible Spectroscopy Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy What is actually being observed spectroscopically is. 1.1 the electromagnetic spectrum 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. To understand why some compounds are colored and others are not, and to determine the relationship. 1.2 wavelength and frequency 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From schematiclistmaxis123.z14.web.core.windows.net
Mechanism Of Uv Visible Spectroscopy Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.2 wavelength and frequency 3. What is actually being observed spectroscopically is. 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Ultraviolet spectroscopy Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy To understand why some compounds are colored and others are not, and to determine the relationship. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. What is actually being observed spectroscopically is. 1.2 wavelength and frequency 3. 1.1 the electromagnetic spectrum 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT UltravioletVisible Spectroscopy PowerPoint Presentation, free Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.1 the electromagnetic spectrum 3. What is actually being observed spectroscopically is. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.2 wavelength and frequency 3. To understand why some compounds are colored and others are not, and to determine the relationship. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.jasco-global.com
Principles of UV/VIS spectroscopy (3) Applications JASCO Global Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy To understand why some compounds are colored and others are not, and to determine the relationship. What is actually being observed spectroscopically is. 1.1 the electromagnetic spectrum 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.2 wavelength and frequency 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.hktechnical.com
UV Visible Spectroscopy Notes PDF B.Pharm 7th Sem Download Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.1 the electromagnetic spectrum 3. What is actually being observed spectroscopically is. 1.2 wavelength and frequency 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. To understand why some compounds are colored and others are not, and to determine the relationship. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From chemistnotes.com
Uv visible spectroscopy basic principle,4 types of shifts,reliable Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy To understand why some compounds are colored and others are not, and to determine the relationship. What is actually being observed spectroscopically is. 1.2 wavelength and frequency 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.1 the electromagnetic spectrum 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.jasco-global.com
Principles of UV/VIS spectroscopy (3) Applications JASCO Global Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. What is actually being observed spectroscopically is. 1.2 wavelength and frequency 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT UVVis spectroscopy PowerPoint Presentation, free download ID Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy To understand why some compounds are colored and others are not, and to determine the relationship. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.1 the electromagnetic spectrum 3. 1.2 wavelength and frequency 3. What is actually being observed spectroscopically is. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT Molecular UVVisible Spectroscopy PowerPoint Presentation ID Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.2 wavelength and frequency 3. What is actually being observed spectroscopically is. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT UVVIS SPECTROSCOPY PowerPoint Presentation, free download ID Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.1 the electromagnetic spectrum 3. What is actually being observed spectroscopically is. To understand why some compounds are colored and others are not, and to determine the relationship. 1.2 wavelength and frequency 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From microbenotes.com
UVVis Spectroscopy Principle, Parts, Uses, Limitations Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy What is actually being observed spectroscopically is. To understand why some compounds are colored and others are not, and to determine the relationship. 1.1 the electromagnetic spectrum 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.2 wavelength and frequency 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.youtube.com
UV / visible Spectroscopy Part2 Electronic Excitation Spectroscopy Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.1 the electromagnetic spectrum 3. What is actually being observed spectroscopically is. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.2 wavelength and frequency 3. To understand why some compounds are colored and others are not, and to determine the relationship. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From psiberg.com
UVVis Spectroscopy Principle, Instrumentation, and Applications Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.1 the electromagnetic spectrum 3. 1.2 wavelength and frequency 3. To understand why some compounds are colored and others are not, and to determine the relationship. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. What is actually being observed spectroscopically is. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From scienceinfo.com
UV Spectroscopy Definition, Principle, Parts, Uses Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.2 wavelength and frequency 3. 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. What is actually being observed spectroscopically is. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT UV / visible Spectroscopy PowerPoint Presentation, free download Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy To understand why some compounds are colored and others are not, and to determine the relationship. 1.1 the electromagnetic spectrum 3. 1.2 wavelength and frequency 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. What is actually being observed spectroscopically is. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From guidelibmisjoinder.z22.web.core.windows.net
Schematic Diagram Of Uv Visible Spectroscopy Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.2 wavelength and frequency 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. What is actually being observed spectroscopically is. To understand why some compounds are colored and others are not, and to determine the relationship. 1.1 the electromagnetic spectrum 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT Electronic (UVvisible) Spectroscopy PowerPoint Presentation Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy What is actually being observed spectroscopically is. 1.2 wavelength and frequency 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. To understand why some compounds are colored and others are not, and to determine the relationship. 1.1 the electromagnetic spectrum 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT UltravioletVisible Spectroscopy PowerPoint Presentation, free Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy What is actually being observed spectroscopically is. 1.2 wavelength and frequency 3. To understand why some compounds are colored and others are not, and to determine the relationship. 1.1 the electromagnetic spectrum 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.youtube.com
Uv spectroscopy/ uvvisible spectroscopy YouTube Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.2 wavelength and frequency 3. What is actually being observed spectroscopically is. To understand why some compounds are colored and others are not, and to determine the relationship. 1.1 the electromagnetic spectrum 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From beingcounsellor.com
The Complete Guide to UV VIS Spectroscopy and How It Can Help with Your Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.2 wavelength and frequency 3. 1.1 the electromagnetic spectrum 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. What is actually being observed spectroscopically is. To understand why some compounds are colored and others are not, and to determine the relationship. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.bartleby.com
UV Visible Spectroscopy bartleby Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.1 the electromagnetic spectrum 3. What is actually being observed spectroscopically is. To understand why some compounds are colored and others are not, and to determine the relationship. 1.2 wavelength and frequency 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT Molecular UVVisible Spectroscopy PowerPoint Presentation ID Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. What is actually being observed spectroscopically is. 1.2 wavelength and frequency 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.slideserve.com
PPT UV/Visible Spectroscopy PowerPoint Presentation, free download Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. What is actually being observed spectroscopically is. 1.1 the electromagnetic spectrum 3. To understand why some compounds are colored and others are not, and to determine the relationship. 1.2 wavelength and frequency 3. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.jasco-global.com
Principles of UV/vis spectroscopy (7) Bandwidth JASCO Global Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy What is actually being observed spectroscopically is. 1.1 the electromagnetic spectrum 3. 1.2 wavelength and frequency 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. To understand why some compounds are colored and others are not, and to determine the relationship. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.vedantu.com
UV Visible Spectroscopy Learn Important Terms and Concepts Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy 1.1 the electromagnetic spectrum 3. The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. What is actually being observed spectroscopically is. 1.2 wavelength and frequency 3. To understand why some compounds are colored and others are not, and to determine the relationship. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.
From www.youtube.com
UV Visible spectroscopy (Instrumentation, working and Applications Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy The absorption of electromagnetic radiations in the uv and visible regions induces the excitation of an electron from a lower to higher molecular. 1.1 the electromagnetic spectrum 3. 1.2 wavelength and frequency 3. To understand why some compounds are colored and others are not, and to determine the relationship. What is actually being observed spectroscopically is. Why Is Uv Visible Spectroscopy Called Electronic Spectroscopy.