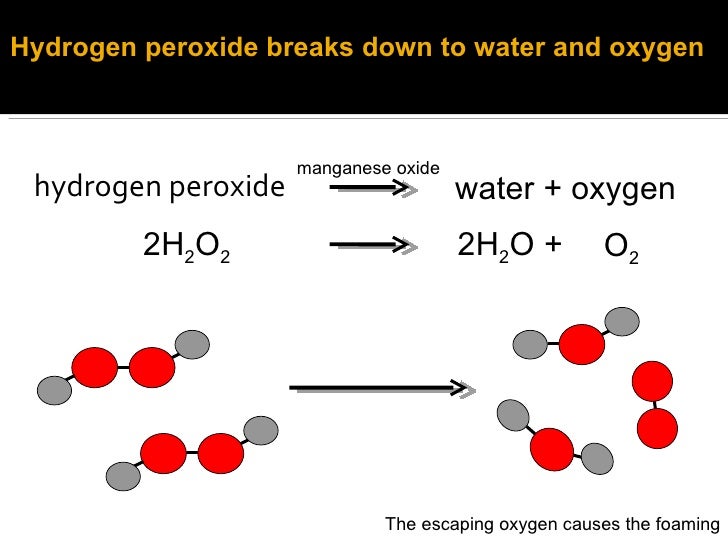
Catalysts For Hydrogen Peroxide Breakdown . The catalase enzyme (found in blood) lowers the. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition.
from www.slideshare.net
As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. The catalase enzyme (found in blood) lowers the. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively.
Effect of Temperature and pH on enzyme activity
Catalysts For Hydrogen Peroxide Breakdown The catalase enzyme (found in blood) lowers the. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalase enzyme (found in blood) lowers the. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively.
From www.slideserve.com
PPT Peroxisomes PowerPoint Presentation ID4802394 Catalysts For Hydrogen Peroxide Breakdown The catalase enzyme (found in blood) lowers the. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world. Catalysts For Hydrogen Peroxide Breakdown.
From www.slideshare.net
Effect of Temperature and pH on enzyme activity Catalysts For Hydrogen Peroxide Breakdown Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead.. Catalysts For Hydrogen Peroxide Breakdown.
From www.semanticscholar.org
Figure 2 from Catalysts for Hydrogen Generation via OxySteam Reforming Catalysts For Hydrogen Peroxide Breakdown The catalase enzyme (found in blood) lowers the. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. A great variety of. Catalysts For Hydrogen Peroxide Breakdown.
From exoukzult.blob.core.windows.net
Possible Catalysts For The Of Hydrogen Peroxide at James Catalysts For Hydrogen Peroxide Breakdown As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. The catalase enzyme (found in blood) lowers the. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. Platinum metal catalysts can. Catalysts For Hydrogen Peroxide Breakdown.
From www.tessshebaylo.com
Hydrogen Peroxide Manganese Dioxide Chemical Equation Tessshebaylo Catalysts For Hydrogen Peroxide Breakdown Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead.. Catalysts For Hydrogen Peroxide Breakdown.
From www.youtube.com
How to Write the Formula for Hydrogen peroxide YouTube Catalysts For Hydrogen Peroxide Breakdown Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. As an interesting contrast, a similar increase in the rate. Catalysts For Hydrogen Peroxide Breakdown.
From www.cell.com
Design of framework catalysts for photocatalytic hydrogen Catalysts For Hydrogen Peroxide Breakdown From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. The catalase enzyme (found in blood) lowers the. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. As an interesting contrast,. Catalysts For Hydrogen Peroxide Breakdown.
From www.mdpi.com
Catalysts Free FullText Catalytic Activation of Hydrogen Peroxide Catalysts For Hydrogen Peroxide Breakdown The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as. Catalysts For Hydrogen Peroxide Breakdown.
From www.researchgate.net
Supramolecular tuning of supported metal phthalocyanine catalysts for Catalysts For Hydrogen Peroxide Breakdown Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalase enzyme (found in blood) lowers the. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. Catalases exert their crucial role in protecting living cells from damage. Catalysts For Hydrogen Peroxide Breakdown.
From www.youtube.com
of of Hydrogen Peroxide Chemical Catalysts For Hydrogen Peroxide Breakdown As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively.. Catalysts For Hydrogen Peroxide Breakdown.
From medium.com
Multiple Uses for Hydrogen Peroxide by Celeste Wilson The DIY Catalysts For Hydrogen Peroxide Breakdown The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. The catalase enzyme (found in blood) lowers the. A great variety of geometries [6] and dimensions of selected supporting catalytic material have. Catalysts For Hydrogen Peroxide Breakdown.
From www.researchgate.net
Scheme II Reaction scheme for the catalytic of hydrogen Catalysts For Hydrogen Peroxide Breakdown Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. The catalytic decomposition of hydrogen. Catalysts For Hydrogen Peroxide Breakdown.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Catalysts For Hydrogen Peroxide Breakdown Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. Catalases exert their crucial role in protecting living cells from damage caused. Catalysts For Hydrogen Peroxide Breakdown.
From chart-studio.plotly.com
Enzyme Catalyzed Rate of Hydrogen Peroxide (H2O2) (Sample Catalysts For Hydrogen Peroxide Breakdown As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal.. Catalysts For Hydrogen Peroxide Breakdown.
From www.researchgate.net
Packinginduced selectivity switching in molecular nanoparticle Catalysts For Hydrogen Peroxide Breakdown The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. The catalase enzyme (found in blood) lowers the. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. As an. Catalysts For Hydrogen Peroxide Breakdown.
From chemistry-europe.onlinelibrary.wiley.com
Co‐based Catalysts for Selective H2O2 Electroproduction via 2‐electron Catalysts For Hydrogen Peroxide Breakdown As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts. Catalysts For Hydrogen Peroxide Breakdown.
From www.youtube.com
Effect of Various Catalysts on Hydrogen Peroxide GCSE Chemistry YouTube Catalysts For Hydrogen Peroxide Breakdown A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. From fresh liver, to powdered manganese, create different catalysts to explore the. Catalysts For Hydrogen Peroxide Breakdown.
From pubs.rsc.org
Insights into alloy/oxide or hydroxide interfaces in NiMobased Catalysts For Hydrogen Peroxide Breakdown Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. The catalytic decomposition of hydrogen. Catalysts For Hydrogen Peroxide Breakdown.
From plotly.com
Average Reaction Rate of Catalase Enzyme in Hydrogen Peroxide bar Catalysts For Hydrogen Peroxide Breakdown From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. Platinum metal catalysts can lower. Catalysts For Hydrogen Peroxide Breakdown.
From 9to5science.com
[Solved] Reaction intermediates of MnO2 catalyzed H2O2 9to5Science Catalysts For Hydrogen Peroxide Breakdown The catalase enzyme (found in blood) lowers the. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. From fresh. Catalysts For Hydrogen Peroxide Breakdown.
From www.mdpi.com
Applied Sciences Free FullText Noble MetalBased Heterogeneous Catalysts For Hydrogen Peroxide Breakdown Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. From fresh liver, to powdered manganese, create different catalysts to explore the. Catalysts For Hydrogen Peroxide Breakdown.
From www.tessshebaylo.com
Hydrogen Peroxide Balanced Chemical Equation Tessshebaylo Catalysts For Hydrogen Peroxide Breakdown Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as. Catalysts For Hydrogen Peroxide Breakdown.
From www.nagwa.com
Question Video Discerning the More Effective Catalyst for Hydrogen Catalysts For Hydrogen Peroxide Breakdown Platinum metal catalysts can lower the activation energy to about 49 kj/mol. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. The catalase enzyme (found in blood) lowers the. The catalytic. Catalysts For Hydrogen Peroxide Breakdown.
From hager-engineering.com
High performance catalysts Hydrogen Peroxide Catalysts Ozone Catalysts For Hydrogen Peroxide Breakdown As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively.. Catalysts For Hydrogen Peroxide Breakdown.
From www.mdpi.com
Catalysts Free FullText The BaeyerVilliger Oxidation of Catalysts For Hydrogen Peroxide Breakdown From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalase enzyme (found in blood) lowers the. A great variety of. Catalysts For Hydrogen Peroxide Breakdown.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID2683513 Catalysts For Hydrogen Peroxide Breakdown As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. The catalase enzyme (found in blood) lowers the. Catalases exert their crucial. Catalysts For Hydrogen Peroxide Breakdown.
From ar.inspiredpencil.com
Catalase Reaction With Hydrogen Peroxide Catalysts For Hydrogen Peroxide Breakdown Catalases exert their crucial role in protecting living cells from damage caused by reactive oxygen species (ros) through effectively. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. As an interesting contrast, a. Catalysts For Hydrogen Peroxide Breakdown.
From www.flinnsci.com
Catalytic of Hydrogen Peroxide Flinn Scientific Catalysts For Hydrogen Peroxide Breakdown As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts. Catalysts For Hydrogen Peroxide Breakdown.
From www.researchgate.net
(PDF) Unveiling the dynamic active site of defective carbonbased Catalysts For Hydrogen Peroxide Breakdown A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead.. Catalysts For Hydrogen Peroxide Breakdown.
From www.pnas.org
Active sites and mechanisms for H2O2 over Pd catalysts PNAS Catalysts For Hydrogen Peroxide Breakdown Platinum metal catalysts can lower the activation energy to about 49 kj/mol. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. Catalases exert. Catalysts For Hydrogen Peroxide Breakdown.
From reubengokegoodwin.blogspot.com
of Hydrogen Peroxide Equation Catalysts For Hydrogen Peroxide Breakdown From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Catalases exert their crucial role in protecting living cells from damage caused. Catalysts For Hydrogen Peroxide Breakdown.
From www.mdpi.com
Catalysts Free FullText Electrocatalytic Oxygen Reduction to Catalysts For Hydrogen Peroxide Breakdown From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. The catalase enzyme (found in blood) lowers the. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. As an interesting contrast,. Catalysts For Hydrogen Peroxide Breakdown.
From www.researchgate.net
(PDF) Photothermalenabled singleatom catalysts for highefficiency Catalysts For Hydrogen Peroxide Breakdown As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead. From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. Catalases exert their crucial role in protecting living cells from damage caused by reactive. Catalysts For Hydrogen Peroxide Breakdown.
From nerdyseal.com
Enzyme Catalase Speeds Up The Breakdown Of Hydrogen Peroxide Into Water Catalysts For Hydrogen Peroxide Breakdown Platinum metal catalysts can lower the activation energy to about 49 kj/mol. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. As an interesting contrast, a similar increase in the rate of decomposition of hydrogen peroxide can be achieved using an inorganic catalyst such as manganese (iv) oxide or lead.. Catalysts For Hydrogen Peroxide Breakdown.
From pubs.acs.org
Progress of Electrochemical Hydrogen Peroxide Synthesis over Single Catalysts For Hydrogen Peroxide Breakdown From fresh liver, to powdered manganese, create different catalysts to explore the effervescent world of hydrogen peroxide. The catalytic decomposition of hydrogen peroxide allows the use of various catalysts that will increase the rate of decomposition. A great variety of geometries [6] and dimensions of selected supporting catalytic material have been studied, ranging from metal. Platinum metal catalysts can lower. Catalysts For Hydrogen Peroxide Breakdown.