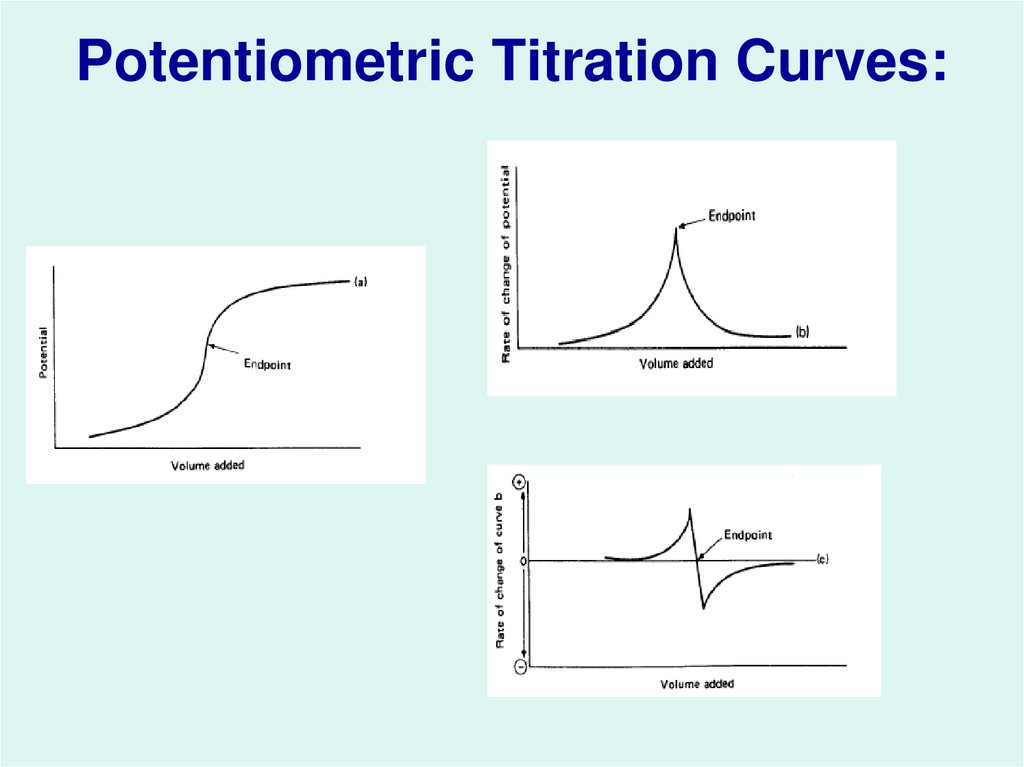
Difference Between Potentiometric Titration And Normal Titration . Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. However, the means of determining the concentration of a species is dependent upon.
from en.ppt-online.org
Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. However, the means of determining the concentration of a species is dependent upon. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes.
Electroanalytical Chemistry online presentation
Difference Between Potentiometric Titration And Normal Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. However, the means of determining the concentration of a species is dependent upon. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis.
From www.differencebetween.com
Difference Between Titration and Back Titration Compare the Difference Between Potentiometric Titration And Normal Titration However, the means of determining the concentration of a species is dependent upon. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Learn. Difference Between Potentiometric Titration And Normal Titration.
From chem.libretexts.org
14.7 AcidBase Titrations Chemistry LibreTexts Difference Between Potentiometric Titration And Normal Titration Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. However, the means of determining the concentration of a species is dependent upon. Learn how to use potentiometry to. Difference Between Potentiometric Titration And Normal Titration.
From www.vrogue.co
Difference Between Acid Base Titration And Redox Titr vrogue.co Difference Between Potentiometric Titration And Normal Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed. Difference Between Potentiometric Titration And Normal Titration.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Difference Between Potentiometric Titration And Normal Titration Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes. Difference Between Potentiometric Titration And Normal Titration.
From www.differencebetween.com
Difference Between Volumetric and Potentiometric Titration Compare Difference Between Potentiometric Titration And Normal Titration Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Potentiometric titration refers to a chemical method of analysis where the endpoint of the. Difference Between Potentiometric Titration And Normal Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Difference Between Potentiometric Titration And Normal Titration Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an. Difference Between Potentiometric Titration And Normal Titration.
From www.differencebetween.com
Difference Between Complexometric and Redox Titration Compare the Difference Between Potentiometric Titration And Normal Titration Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration. Difference Between Potentiometric Titration And Normal Titration.
From www.numerade.com
SOLVED 2 What is the true regarding to the basic principle of Difference Between Potentiometric Titration And Normal Titration Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an. Difference Between Potentiometric Titration And Normal Titration.
From www.purechemistry.org
ACIDBASE TITRATION Purechemistry Difference Between Potentiometric Titration And Normal Titration Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the. Difference Between Potentiometric Titration And Normal Titration.
From www.aiophotoz.com
Difference Between Acid Base Titration And Redox Titration Acid Base Difference Between Potentiometric Titration And Normal Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in. Difference Between Potentiometric Titration And Normal Titration.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration Difference Between Potentiometric Titration And Normal Titration Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. However, the means of determining the concentration of a species is dependent upon. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn how. Difference Between Potentiometric Titration And Normal Titration.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID6718194 Difference Between Potentiometric Titration And Normal Titration Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to. Difference Between Potentiometric Titration And Normal Titration.
From www.differencebetween.com
Difference Between Volumetric and Potentiometric Titration Compare Difference Between Potentiometric Titration And Normal Titration Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. However, the means of determining the concentration of a species is dependent upon. Learn about potentiometric titration, a. Difference Between Potentiometric Titration And Normal Titration.
From www.studyread.com
Potentiometric Titration with Its Principle, Applications and Advanatages Difference Between Potentiometric Titration And Normal Titration Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Learn about potentiometric titration, a method to determine the concentration of an analyte by. Difference Between Potentiometric Titration And Normal Titration.
From www.differencebetween.com
Difference Between Potentiometric and Conductometric Titrations Difference Between Potentiometric Titration And Normal Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it. Difference Between Potentiometric Titration And Normal Titration.
From en.ppt-online.org
Electroanalytical Chemistry online presentation Difference Between Potentiometric Titration And Normal Titration Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. However, the means of determining the concentration of a species is dependent upon. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn about different types of. Difference Between Potentiometric Titration And Normal Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Difference Between Potentiometric Titration And Normal Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential. Difference Between Potentiometric Titration And Normal Titration.
From www.researchgate.net
Potentiometric titration of CRM Batch 30. Plot of the difference Difference Between Potentiometric Titration And Normal Titration Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. However, the means of determining the concentration of a species is dependent upon. Learn how to. Difference Between Potentiometric Titration And Normal Titration.
From www.researchgate.net
4 Potentiometric titration of a solution containing a weak acid (CH 3 Difference Between Potentiometric Titration And Normal Titration Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Potentiometric titration is a technique that relies on the measurement of the potential difference. Difference Between Potentiometric Titration And Normal Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Difference Between Potentiometric Titration And Normal Titration However, the means of determining the concentration of a species is dependent upon. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn about different types. Difference Between Potentiometric Titration And Normal Titration.
From www.researchgate.net
Typical curves of potentiometric titrations starting from the PZC Difference Between Potentiometric Titration And Normal Titration Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. However, the means of determining the concentration of a species is dependent upon. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn how to use potentiometry to. Difference Between Potentiometric Titration And Normal Titration.
From pediaa.com
Difference Between Back Titration and Direct Titration Definition Difference Between Potentiometric Titration And Normal Titration Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. However, the means of determining the concentration of a species is dependent upon. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn how to use potentiometry to. Difference Between Potentiometric Titration And Normal Titration.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Difference Between Potentiometric Titration And Normal Titration Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is. Difference Between Potentiometric Titration And Normal Titration.
From thenoveldifference.com
Volumetric and Potentiometric Titration best 5 difference Difference Between Potentiometric Titration And Normal Titration Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used. Difference Between Potentiometric Titration And Normal Titration.
From hxeumtgfn.blob.core.windows.net
Difference Between Titration And Buffer at Eula Bell blog Difference Between Potentiometric Titration And Normal Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. However, the means of determining the concentration of a species is dependent upon. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn about different types of titrations,. Difference Between Potentiometric Titration And Normal Titration.
From www.scribd.com
Potentiometric Titration Titration Buffer Solution Difference Between Potentiometric Titration And Normal Titration Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. However, the means of determining the concentration of a species is dependent upon. Learn about different types. Difference Between Potentiometric Titration And Normal Titration.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 Difference Between Potentiometric Titration And Normal Titration Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is. Difference Between Potentiometric Titration And Normal Titration.
From www.differencebetween.com
Difference Between Volumetric and Potentiometric Titration Compare Difference Between Potentiometric Titration And Normal Titration Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are. Difference Between Potentiometric Titration And Normal Titration.
From www.researchgate.net
Photometric and potentiometric titration curves of (a) 25.00 mL of Difference Between Potentiometric Titration And Normal Titration Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. However, the means of determining the concentration of a species is dependent upon. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Learn how to. Difference Between Potentiometric Titration And Normal Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Difference Between Potentiometric Titration And Normal Titration Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes. Difference Between Potentiometric Titration And Normal Titration.
From thenoveldifference.com
Volumetric and Potentiometric Titration best 5 difference Difference Between Potentiometric Titration And Normal Titration Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Potentiometric titration is a technique that relies on the measurement of the potential difference. Difference Between Potentiometric Titration And Normal Titration.
From www.differencebetween.com
Difference Between Potentiometric and Conductometric Titrations Difference Between Potentiometric Titration And Normal Titration Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to quantitative analysis. Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential. Difference Between Potentiometric Titration And Normal Titration.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare Difference Between Potentiometric Titration And Normal Titration Potentiometric titration is a technique that relies on the measurement of the potential difference between two electrodes immersed in the. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two. Difference Between Potentiometric Titration And Normal Titration.
From thenoveldifference.com
Volumetric and Potentiometric Titration best 5 difference Difference Between Potentiometric Titration And Normal Titration Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration. Difference Between Potentiometric Titration And Normal Titration.
From www.youtube.com
Iodometric vs Iodimetric Titrations Basics Redox Titration viva of Difference Between Potentiometric Titration And Normal Titration However, the means of determining the concentration of a species is dependent upon. Learn about different types of titrations, such as classical redox, coulometric and amperometric, and how they are used to determine the concentration of analytes. Learn about potentiometric titration, a method to determine the concentration of an analyte by measuring the electric potential across two electrodes. Learn how. Difference Between Potentiometric Titration And Normal Titration.