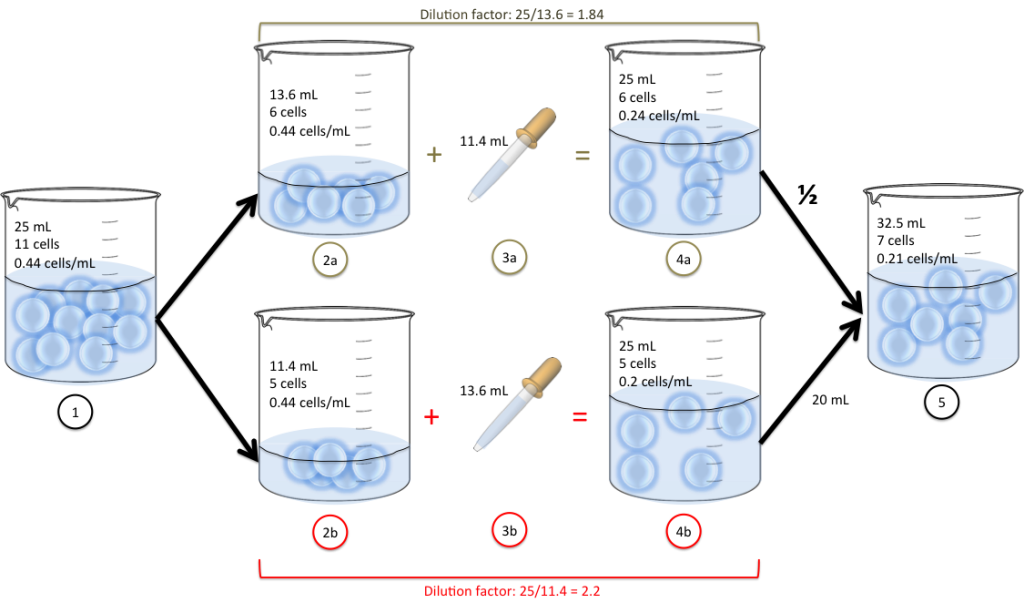
Does Dilution Factor Have Units . In both dilution and concentration,. Df = v f v i = 10.0ml 0.1ml = 100. State whether the concentration of a solution is directly or indirectly proportional to its volume. Note that a 1:20 quotient can represent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A solution is produced when a solute dissolves in a solvent. Dilution means to reduce the concentration of a solution. If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. Dilution factor is the factor by which the stock solution is diluted. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml.
from www.hemocytometer.org
State whether the concentration of a solution is directly or indirectly proportional to its volume. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution means to reduce the concentration of a solution. Dilution factor is the factor by which the stock solution is diluted. Df = v f v i = 10.0ml 0.1ml = 100. A solution is produced when a solute dissolves in a solvent. In both dilution and concentration,. Note that a 1:20 quotient can represent. If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,.
Using the dilution factor to calculate dilutions • Hemocytometer
Does Dilution Factor Have Units State whether the concentration of a solution is directly or indirectly proportional to its volume. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. A solution is produced when a solute dissolves in a solvent. If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. State whether the concentration of a solution is directly or indirectly proportional to its volume. Note that a 1:20 quotient can represent. Dilution means to reduce the concentration of a solution. In both dilution and concentration,. Df = v f v i = 10.0ml 0.1ml = 100. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution factor is the factor by which the stock solution is diluted.
From klapssgkb.blob.core.windows.net
Dilution Series Of Cells at Brenda Ward blog Does Dilution Factor Have Units Dilution factor is the factor by which the stock solution is diluted. A solution is produced when a solute dissolves in a solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,.. Does Dilution Factor Have Units.
From www.youtube.com
Dilution factor video YouTube Does Dilution Factor Have Units A solution is produced when a solute dissolves in a solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of. Does Dilution Factor Have Units.
From www.slideserve.com
PPT MCB 151 PowerPoint Presentation, free download ID5761124 Does Dilution Factor Have Units If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. Note that a 1:20 quotient can represent. State whether the concentration of a solution is directly or indirectly proportional to its volume. In both dilution and concentration,. Dilution means to reduce the concentration of a solution. V f =. Does Dilution Factor Have Units.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Does Dilution Factor Have Units State whether the concentration of a solution is directly or indirectly proportional to its volume. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution factor. Does Dilution Factor Have Units.
From klafwpnrs.blob.core.windows.net
Dilution Formula Explanation at John Schell blog Does Dilution Factor Have Units V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution means to reduce the concentration of a solution. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. Df = v f v i = 10.0ml. Does Dilution Factor Have Units.
From www.slideshare.net
Molarity and dilution Does Dilution Factor Have Units Dilution factor is the factor by which the stock solution is diluted. State whether the concentration of a solution is directly or indirectly proportional to its volume. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Note that a 1:20 quotient can represent. In both dilution and concentration,. A solution is produced when. Does Dilution Factor Have Units.
From www.studocu.com
Calculating Dilution Factors Worksheet(1) Tagged How to Perform Does Dilution Factor Have Units Dilution means to reduce the concentration of a solution. Note that a 1:20 quotient can represent. In both dilution and concentration,. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A solution is produced when a solute. Does Dilution Factor Have Units.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Does Dilution Factor Have Units Df = v f v i = 10.0ml 0.1ml = 100. A solution is produced when a solute dissolves in a solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution means to reduce the concentration of a solution. If you wish to perform dilution factor or fold dilution calculations for solutions. Does Dilution Factor Have Units.
From www.youtube.com
How to Calculate CFUSerial Dilution Microbiology Technique knowledge Does Dilution Factor Have Units State whether the concentration of a solution is directly or indirectly proportional to its volume. In both dilution and concentration,. Dilution means to reduce the concentration of a solution. If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. A 1:20 dilution factor means that for each unit of. Does Dilution Factor Have Units.
From support.axionbio.com
Understanding of Cell Sample Dilution Factor Does Dilution Factor Have Units Df = v f v i = 10.0ml 0.1ml = 100. In both dilution and concentration,. Dilution means to reduce the concentration of a solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution factor is. Does Dilution Factor Have Units.
From www.youtube.com
How to do Dilution and Serial Dilution? Dilution Factor Calculations Does Dilution Factor Have Units A solution is produced when a solute dissolves in a solvent. If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Note that a 1:20 quotient can represent. Dilution factor is the factor. Does Dilution Factor Have Units.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Does Dilution Factor Have Units State whether the concentration of a solution is directly or indirectly proportional to its volume. A solution is produced when a solute dissolves in a solvent. Note that a 1:20 quotient can represent. Dilution factor is the factor by which the stock solution is diluted. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0. Does Dilution Factor Have Units.
From www.freepik.com
Premium Vector Dilution factor formula science vector illustration Does Dilution Factor Have Units A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. Dilution means to reduce the concentration of a solution. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution factor is. Does Dilution Factor Have Units.
From klaivqdcu.blob.core.windows.net
What Does Dilute In Chemistry Mean at Sharron Wallace blog Does Dilution Factor Have Units Dilution means to reduce the concentration of a solution. In both dilution and concentration,. Df = v f v i = 10.0ml 0.1ml = 100. If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml =. Does Dilution Factor Have Units.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Does Dilution Factor Have Units Df = v f v i = 10.0ml 0.1ml = 100. Dilution means to reduce the concentration of a solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A solution is produced when a solute dissolves in a solvent. Note that a 1:20 quotient can represent. V f = aliquot volume +. Does Dilution Factor Have Units.
From cepsppxa.blob.core.windows.net
How To Calculate Dilution Factor In Spectrophotometer at Troy Cox blog Does Dilution Factor Have Units A solution is produced when a solute dissolves in a solvent. Dilution factor is the factor by which the stock solution is diluted. Note that a 1:20 quotient can represent. In both dilution and concentration,. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of. Does Dilution Factor Have Units.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Does Dilution Factor Have Units Note that a 1:20 quotient can represent. Dilution means to reduce the concentration of a solution. Dilution factor is the factor by which the stock solution is diluted. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. If you wish to perform dilution factor or fold dilution calculations for solutions with units per. Does Dilution Factor Have Units.
From www.youtube.com
How Do You Calculate Dilution Factor? YouTube Does Dilution Factor Have Units State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution factor is the factor by which the stock solution is diluted. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. Dilution is the addition of solvent,. Does Dilution Factor Have Units.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer Does Dilution Factor Have Units A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. Dilution means to reduce the concentration of a solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A solution is produced when a solute dissolves. Does Dilution Factor Have Units.
From www.pdfprof.com
how to calculate dilution factor Does Dilution Factor Have Units Dilution means to reduce the concentration of a solution. Note that a 1:20 quotient can represent. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. State whether the concentration of a solution is directly or indirectly proportional to its volume. In both. Does Dilution Factor Have Units.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Does Dilution Factor Have Units If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. Df = v f v i = 10.0ml 0.1ml = 100. A solution is produced when a solute dissolves in a solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. Note that. Does Dilution Factor Have Units.
From exonuljgk.blob.core.windows.net
Cell Culture Dilution Calculator at Leslie Franks blog Does Dilution Factor Have Units State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution means to reduce the concentration of a solution. Df = v f v i = 10.0ml 0.1ml = 100. In both dilution and concentration,. Dilution factor is the factor by which the stock solution is diluted. Note that a 1:20 quotient can represent. If. Does Dilution Factor Have Units.
From lasopamail784.weebly.com
Calculate Dilution Factor lasopamail Does Dilution Factor Have Units A solution is produced when a solute dissolves in a solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. Df = v f v i = 10.0ml 0.1ml = 100. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Note that a 1:20 quotient can represent.. Does Dilution Factor Have Units.
From www.scribd.com
DilutionFactor Does Dilution Factor Have Units Note that a 1:20 quotient can represent. Df = v f v i = 10.0ml 0.1ml = 100. Dilution factor is the factor by which the stock solution is diluted. In both dilution and concentration,. If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. State whether the concentration. Does Dilution Factor Have Units.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer Does Dilution Factor Have Units V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. Df = v f v i = 10.0ml 0.1ml = 100. Note that a 1:20 quotient can. Does Dilution Factor Have Units.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Does Dilution Factor Have Units If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution factor is the factor by which the stock solution is diluted. A solution is produced when a solute dissolves in a solvent.. Does Dilution Factor Have Units.
From www.youtube.com
How to Calculate Dilution Factor YouTube Does Dilution Factor Have Units A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. Dilution means to reduce the concentration of a solution. Df = v f v i = 10.0ml 0.1ml = 100. A solution is produced when a solute dissolves in a solvent. Dilution factor. Does Dilution Factor Have Units.
From exofbishk.blob.core.windows.net
Difference Between Dilution Factor And Dilution Ratio at Harriett Does Dilution Factor Have Units A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. In both dilution and concentration,. Df = v f v i = 10.0ml 0.1ml = 100. Dilution factor is the factor by which the stock solution is diluted. Dilution is the addition of. Does Dilution Factor Have Units.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Does Dilution Factor Have Units A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. In both dilution and concentration,. If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. Note that a 1:20 quotient can represent.. Does Dilution Factor Have Units.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Does Dilution Factor Have Units A solution is produced when a solute dissolves in a solvent. Df = v f v i = 10.0ml 0.1ml = 100. Note that a 1:20 quotient can represent. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. If you wish to. Does Dilution Factor Have Units.
From www.youtube.com
Calculating Dilution Factor YouTube Does Dilution Factor Have Units Df = v f v i = 10.0ml 0.1ml = 100. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. In both dilution and concentration,. Dilution factor is the factor by which the stock solution is diluted. V f = aliquot volume. Does Dilution Factor Have Units.
From www.slideserve.com
PPT Serial Dilutions PowerPoint Presentation, free download ID149795 Does Dilution Factor Have Units Dilution means to reduce the concentration of a solution. In both dilution and concentration,. Dilution factor is the factor by which the stock solution is diluted. Df = v f v i = 10.0ml 0.1ml = 100. A solution is produced when a solute dissolves in a solvent. Note that a 1:20 quotient can represent. State whether the concentration of. Does Dilution Factor Have Units.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Does Dilution Factor Have Units A solution is produced when a solute dissolves in a solvent. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution means to reduce the concentration of a solution. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume. Does Dilution Factor Have Units.
From www.pdfprof.com
how to calculate dilution factor Does Dilution Factor Have Units Note that a 1:20 quotient can represent. A 1:20 dilution factor means that for each unit of the stock solution, there are 19 units of dilutant, resulting in a total volume of 20 units. A solution is produced when a solute dissolves in a solvent. Df = v f v i = 10.0ml 0.1ml = 100. In both dilution and. Does Dilution Factor Have Units.
From denisseqibowen.blogspot.com
How to Calculate Dilution Factor DenisseqiBowen Does Dilution Factor Have Units Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. If you wish to perform dilution factor or fold dilution calculations for solutions with units per volume concentration units (e.g., mu/ml,. Df = v f v i = 10.0ml 0.1ml = 100. A 1:20 dilution factor means that for each unit of the stock. Does Dilution Factor Have Units.