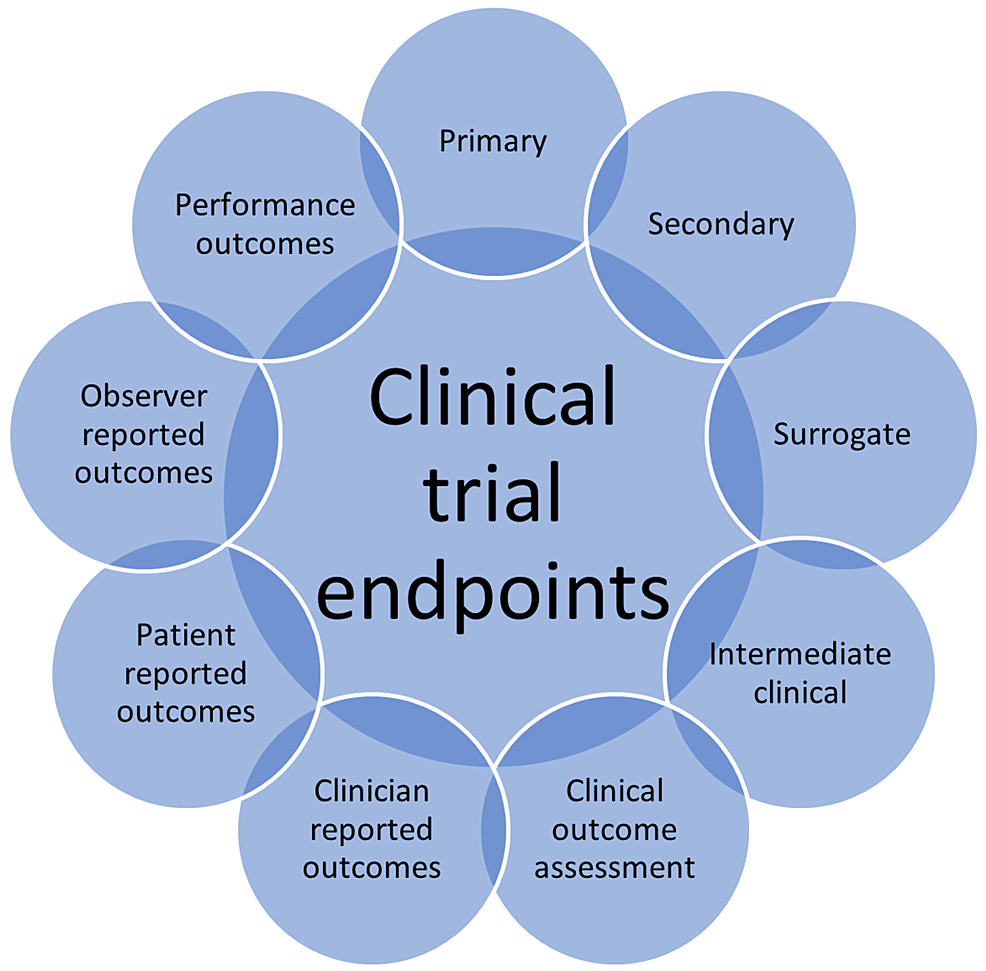
Endpoints Research . A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. Often these measures are combined together. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy being studied. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. Endpoints are the key measures used to determine the success or failure of a clinical trial. Endpoints are measures which can be observed or calculated for each subject on a trial.
from www.cureus.com
Endpoints are measures which can be observed or calculated for each subject on a trial. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. Often these measures are combined together. Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy being studied. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. Endpoints are the key measures used to determine the success or failure of a clinical trial.
Research Question, Objectives, and Endpoints in Clinical and
Endpoints Research A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. Often these measures are combined together. Endpoints are measures which can be observed or calculated for each subject on a trial. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. Endpoints are the key measures used to determine the success or failure of a clinical trial. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy being studied.
From www.tldrpharmacy.com
A Guide to Clinical Trial Endpoints — tl;dr pharmacy Endpoints Research Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy being studied. They are the primary outcomes researchers use to evaluate the efficacy and safety. Endpoints Research.
From www.researchgate.net
Study design and primary and secondary endpoints 208 children with Endpoints Research Endpoints are measures which can be observed or calculated for each subject on a trial. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. Often these measures are combined together. Understanding the range of endpoints available for use and the context in which they have arisen together with. Endpoints Research.
From blog.theactigraph.com
ActiGraph Announces Grant to Accelerate Digital Endpoint Research Endpoints Research A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. Often these measures are combined together. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the. Endpoints Research.
From www.researchgate.net
Research endpoints for the evaluation of POCT used in outpatient and Endpoints Research Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory. Endpoints Research.
From www.researchgate.net
Primary and secondary study endpoints and corresponding Endpoints Research They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. Endpoints. Endpoints Research.
From www.microsoft.com
Microsoft is recognized as a Leader in the 2021 Forrester Wave for Endpoints Research Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single. Endpoints Research.
From www.slideserve.com
PPT Fundamentals of Clinical Trials Endpoints Betsy Garofalo MD Endpoints Research Endpoints are the key measures used to determine the success or failure of a clinical trial. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy being studied. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. A quality. Endpoints Research.
From cra-school.com
The Endpoint Selection a Complex Process in the Clinical Trials Design Endpoints Research In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. Understanding the range of endpoints available for use and the context in which they have arisen together. Endpoints Research.
From www.researchgate.net
(PDF) A review on humane endpoints in animal experimentation for Endpoints Research They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. Endpoints. Endpoints Research.
From pharmrev.aspetjournals.org
Development of Novel, ValueBased, Digital Endpoints for Clinical Endpoints Research Endpoints are measures which can be observed or calculated for each subject on a trial. Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy. Endpoints Research.
From www.cureus.com
Cureus Research Question, Objectives, and Endpoints in Clinical and Endpoints Research Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single. Endpoints Research.
From www.slideserve.com
PPT Multiple Primary Endpoints in Clinical Trials PowerPoint Endpoints Research Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. An endpoint. Endpoints Research.
From www.slideteam.net
Clinical Trial Phases With Endpoints And Timings Clinical Research Endpoints Research They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. Endpoints are the key measures used to determine the success or failure of a clinical trial. Endpoints are measures which can be observed. Endpoints Research.
From www.slideshare.net
Endpoints in clinical research Endpoints Research Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. Endpoints are measures which can be observed or calculated for each subject on a trial. A quality outcome or endpoint will be clinically. Endpoints Research.
From www.cureus.com
Research Question, Objectives, and Endpoints in Clinical and Endpoints Research A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. Understanding the range of endpoints available for use and the context in which they have arisen together. Endpoints Research.
From william-has-bryant.blogspot.com
Endpoint Criteria Describe When It Is Time to Do What WilliamhasBryant Endpoints Research They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. Often these measures are combined together. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. Endpoints are the key measures used to determine the success or failure of a clinical trial.. Endpoints Research.
From www.powershow.com
PPT Surrogate Endpoints in Clinical Research. Clinical Trials Endpoints Research Often these measures are combined together. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. Endpoints are measures which can be observed or calculated for each subject on a trial. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory. Endpoints Research.
From www.bmc.com
Introduction to Endpoints Benefits and Use Cases BMC Software Blogs Endpoints Research Endpoints are the key measures used to determine the success or failure of a clinical trial. Often these measures are combined together. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. Endpoints are measures which can be observed or calculated for each subject on a trial. They are. Endpoints Research.
From www.thelancet.com
and endpoints in cancer trials bridging the divide The Endpoints Research An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy being studied. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory. Endpoints Research.
From www.clinixir.com
Unleashing Potential for Digital Endpoints How They Can Revolutionize Endpoints Research Endpoints are the key measures used to determine the success or failure of a clinical trial. Endpoints are measures which can be observed or calculated for each subject on a trial. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. An endpoint is a targeted outcome of a. Endpoints Research.
From www.researchgate.net
(PDF) When and How Can Endpoints Be Changed after Initiation of a Endpoints Research Often these measures are combined together. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine. Endpoints Research.
From www.researchgate.net
(PDF) Factors Influencing Depression Endpoints Research (FINDER Endpoints Research Endpoints are the key measures used to determine the success or failure of a clinical trial. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy being studied. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single. Endpoints Research.
From www.slideserve.com
PPT Humane endpoints and use of score sheets PowerPoint Presentation Endpoints Research Endpoints are the key measures used to determine the success or failure of a clinical trial. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of. Endpoints Research.
From delvehealth.com
Digital Endpoints Have a Positive Effect on Clinical Trials Clinical Endpoints Research Often these measures are combined together. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine. Endpoints Research.
From www.slideserve.com
PPT What are Important Endpoints in Anaesthesia Research? PowerPoint Endpoints Research Endpoints are measures which can be observed or calculated for each subject on a trial. Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. In the realm. Endpoints Research.
From www.iqvia.com
Evolving Oncology Endpoints IQVIA Endpoints Research An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy being studied. Often these measures are combined together. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. Endpoints are the key measures used. Endpoints Research.
From www.researchgate.net
Primary and secondary endpoints of the study. Download Table Endpoints Research Endpoints are measures which can be observed or calculated for each subject on a trial. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. Often these measures are combined together. An endpoint. Endpoints Research.
From www.researchgate.net
(PDF) Development of Novel, ValueBased, Digital Endpoints for Clinical Endpoints Research A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. Understanding the range of endpoints available for use and the context in which they have arisen together with their strengths and. Endpoints are measures which can be observed or calculated for each subject on a trial. An endpoint is. Endpoints Research.
From www.bloorresearch.com
The importance of endpoints to security Bloor Research Endpoints Research A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy being studied. Often these measures are combined together. They are the primary outcomes researchers use. Endpoints Research.
From www.researchgate.net
(PDF) Endpoints in Clinical Trials Advantages and Limitations Endpoints Research Endpoints are the key measures used to determine the success or failure of a clinical trial. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. Endpoints are measures which can be observed or calculated for each subject on a trial. Understanding the range of endpoints available for use and the context in which. Endpoints Research.
From www.sentinelone.com
What is Endpoint Management? Policies and Solutions Endpoints Research In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. A quality outcome or endpoint will be clinically meaningful, straightforward to collect, and will be sensitive to address research questions of. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. An. Endpoints Research.
From www.researchgate.net
Study endpoints and definitions Study endpoints Primary endpoint Endpoints Research In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. Endpoints are the key measures used to determine the success or failure of a clinical trial. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. An endpoint is a targeted outcome. Endpoints Research.
From www.researchgate.net
1 The application domain of humane endpoints. (Adapted from Richmond Endpoints Research Endpoints are measures which can be observed or calculated for each subject on a trial. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. In the realm of clinical trials, the selection of appropriate endpoints is a cornerstone that determines the trajectory and outcome of the. Understanding the range of endpoints available for. Endpoints Research.
From endpoints.eu
Our Research ENDpoiNTS Endpoints Research Endpoints are measures which can be observed or calculated for each subject on a trial. An endpoint is a targeted outcome of a clinical trial that is statistically analyzed to help determine the efficacy and safety of the therapy being studied. Often these measures are combined together. Endpoints are the key measures used to determine the success or failure of. Endpoints Research.
From www.researchgate.net
Definition of Primary Endpoint Bleeding Academic Research Consortium Endpoints Research Endpoints are measures which can be observed or calculated for each subject on a trial. Often these measures are combined together. A composite endpoint (cep) is an outcome that combines two or more endpoints of interest within a single variable [1, 2]. They are the primary outcomes researchers use to evaluate the efficacy and safety of a new. Understanding the. Endpoints Research.