Krypton And Fluorine Reaction . krf 2 is an extremely strong oxidizing and fluorinating agent. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. It can convert metallic gold to auf 5, metallic. why can fluorine react with krypton to form a compound? What is the electron configuration of krypton? reaction of fluorine with noble gasses. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. Stable compounds of xenon form when. What colors are most pronounced in the.
from www.frontiersin.org
What is the electron configuration of krypton? krf 2 is an extremely strong oxidizing and fluorinating agent. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. It can convert metallic gold to auf 5, metallic. why can fluorine react with krypton to form a compound? What colors are most pronounced in the. reaction of fluorine with noble gasses. Stable compounds of xenon form when. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta.
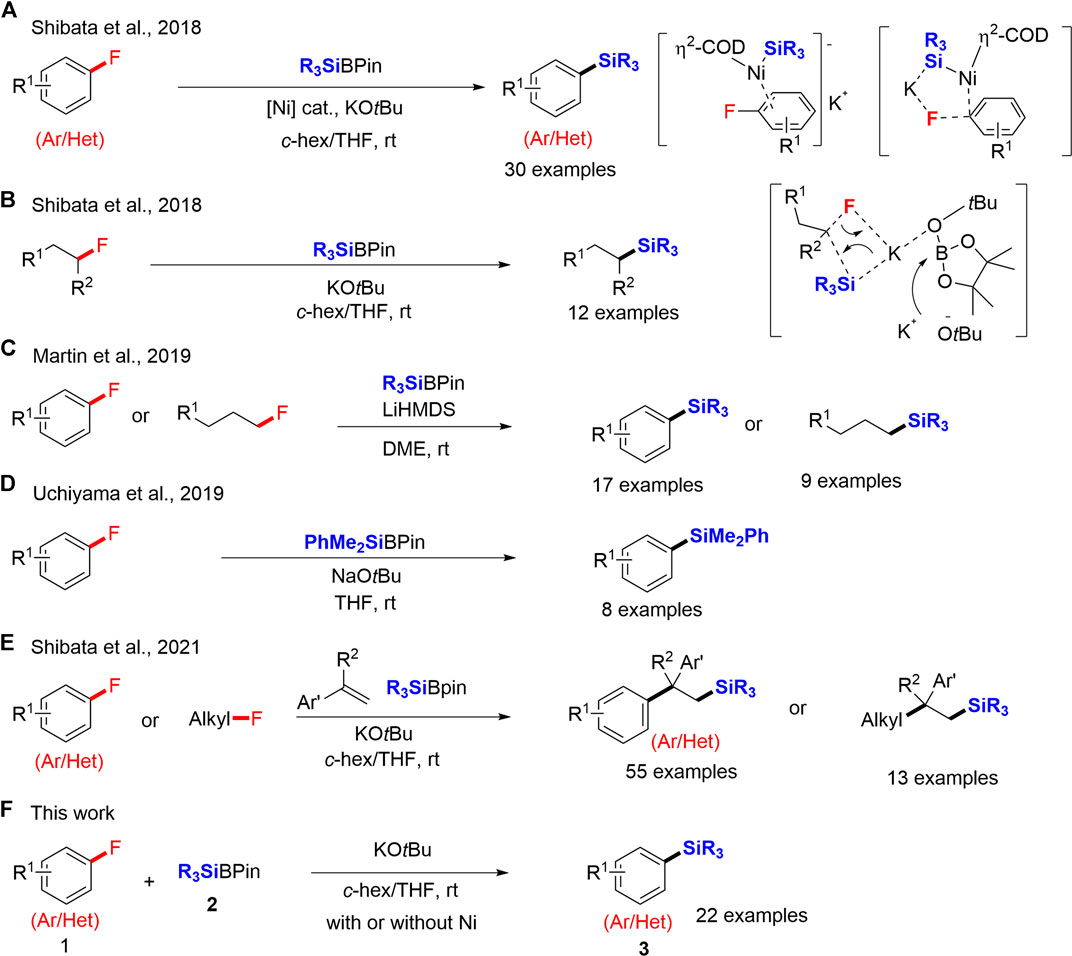
Frontiers SilylboronateMediated Defluorosilylation of Aryl Fluorides
Krypton And Fluorine Reaction What is the electron configuration of krypton? Stable compounds of xenon form when. What colors are most pronounced in the. It can convert metallic gold to auf 5, metallic. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. What is the electron configuration of krypton? reaction of fluorine with noble gasses. krf 2 is an extremely strong oxidizing and fluorinating agent. why can fluorine react with krypton to form a compound? krypton forms a difluoride, krf 2, which is thermally unstable at room temperature.
From www.numerade.com
SOLVED 'Solid potassium and fluorine gas are produced by the Krypton And Fluorine Reaction Stable compounds of xenon form when. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. why can fluorine react with krypton to form a compound? What colors are most pronounced in the. What is the electron configuration of krypton? krf 2 is an extremely strong oxidizing and fluorinating agent. It can convert metallic. Krypton And Fluorine Reaction.
From festivalegiptoenbarcelona.com
Símbolos y estructuras de Lewis CHEM 1305 Introductory Chemistry El Krypton And Fluorine Reaction It can convert metallic gold to auf 5, metallic. why can fluorine react with krypton to form a compound? What is the electron configuration of krypton? krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. reaction of fluorine with noble gasses. What colors are most pronounced in the. krf 2 is an. Krypton And Fluorine Reaction.
From www.f3enc.com
Activation Energy F3 ENC Crossbones Krypton And Fluorine Reaction What colors are most pronounced in the. What is the electron configuration of krypton? in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. krf 2 is an extremely strong oxidizing and fluorinating agent. Stable compounds of xenon form when. reaction of fluorine with noble gasses. It can convert. Krypton And Fluorine Reaction.
From www.toppr.com
Fluorine reacts with water to give Krypton And Fluorine Reaction Stable compounds of xenon form when. why can fluorine react with krypton to form a compound? It can convert metallic gold to auf 5, metallic. What is the electron configuration of krypton? krf 2 is an extremely strong oxidizing and fluorinating agent. reaction of fluorine with noble gasses. krypton forms a difluoride, krf 2, which is. Krypton And Fluorine Reaction.
From www.dreamstime.com
Sodium Fluoride Ionic Compound Created Stock Vector Illustration of Krypton And Fluorine Reaction krf 2 is an extremely strong oxidizing and fluorinating agent. What colors are most pronounced in the. reaction of fluorine with noble gasses. It can convert metallic gold to auf 5, metallic. why can fluorine react with krypton to form a compound? Stable compounds of xenon form when. krypton forms a difluoride, krf 2, which is. Krypton And Fluorine Reaction.
From sites.google.com
CS102B(II) Elements, Compounds and Mixtures SOTA Scientific Krypton And Fluorine Reaction Stable compounds of xenon form when. why can fluorine react with krypton to form a compound? krf 2 is an extremely strong oxidizing and fluorinating agent. It can convert metallic gold to auf 5, metallic. What colors are most pronounced in the. What is the electron configuration of krypton? in each method, the highly reactive fluorine radicals. Krypton And Fluorine Reaction.
From www.frontiersin.org
Frontiers SilylboronateMediated Defluorosilylation of Aryl Fluorides Krypton And Fluorine Reaction What is the electron configuration of krypton? why can fluorine react with krypton to form a compound? in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. Stable compounds of xenon form when. It can convert metallic gold to auf 5, metallic. krf 2 is an extremely strong oxidizing. Krypton And Fluorine Reaction.
From www.numerade.com
SOLVEDFluorine Beta Phase Fluorine can crystalize into a socalled Krypton And Fluorine Reaction It can convert metallic gold to auf 5, metallic. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. reaction of fluorine with noble gasses. why can fluorine react with krypton to form a compound? Stable compounds of xenon form when. What is the electron configuration of krypton? . Krypton And Fluorine Reaction.
From www.researchgate.net
Scheme 62. Silver promoted fluorotrifluoromethylation of alkenes Krypton And Fluorine Reaction It can convert metallic gold to auf 5, metallic. krf 2 is an extremely strong oxidizing and fluorinating agent. What colors are most pronounced in the. What is the electron configuration of krypton? in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. Stable compounds of xenon form when. . Krypton And Fluorine Reaction.
From dokumen.tips
(PDF) Photochemical Formation of Krypton Difluoride from Krypton and Krypton And Fluorine Reaction krf 2 is an extremely strong oxidizing and fluorinating agent. What colors are most pronounced in the. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. why can fluorine react with krypton to form a compound? It can convert metallic gold to auf 5, metallic. reaction of. Krypton And Fluorine Reaction.
From pubs.rsc.org
The unique fluorine effects in organic reactions recent facts and Krypton And Fluorine Reaction krf 2 is an extremely strong oxidizing and fluorinating agent. It can convert metallic gold to auf 5, metallic. reaction of fluorine with noble gasses. Stable compounds of xenon form when. What colors are most pronounced in the. What is the electron configuration of krypton? why can fluorine react with krypton to form a compound? krypton. Krypton And Fluorine Reaction.
From thepowerofkrypton.weebly.com
COMPOUNDS THE POWER OF KRYPTON Krypton And Fluorine Reaction It can convert metallic gold to auf 5, metallic. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. Stable compounds of xenon form when. reaction of fluorine with noble gasses. krf 2 is an extremely strong oxidizing and fluorinating agent. What is the electron configuration of krypton? why can fluorine react with. Krypton And Fluorine Reaction.
From www.chegg.com
Solved 19. [7 pts] Krypton and fluorine combine to form Krypton And Fluorine Reaction Stable compounds of xenon form when. What colors are most pronounced in the. What is the electron configuration of krypton? reaction of fluorine with noble gasses. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature.. Krypton And Fluorine Reaction.
From periodictable.com
Poster sample, a sample of the element Fluorine in the Periodic Table Krypton And Fluorine Reaction why can fluorine react with krypton to form a compound? What colors are most pronounced in the. krf 2 is an extremely strong oxidizing and fluorinating agent. Stable compounds of xenon form when. reaction of fluorine with noble gasses. It can convert metallic gold to auf 5, metallic. krypton forms a difluoride, krf 2, which is. Krypton And Fluorine Reaction.
From www.youtube.com
Fluorine reactions YouTube Krypton And Fluorine Reaction why can fluorine react with krypton to form a compound? in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. What colors are most pronounced in the. What is the electron configuration of krypton? It can convert metallic gold to auf 5, metallic. reaction of fluorine with noble gasses.. Krypton And Fluorine Reaction.
From cen.acs.org
FluorineChemistryContinuesApace Krypton And Fluorine Reaction It can convert metallic gold to auf 5, metallic. What colors are most pronounced in the. why can fluorine react with krypton to form a compound? in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. krf 2 is an extremely strong oxidizing and fluorinating agent. What is the. Krypton And Fluorine Reaction.
From techiescientist.com
KrF2 Lewis Structure, Hybridization, Molecular Geometry, and Polarity Krypton And Fluorine Reaction reaction of fluorine with noble gasses. It can convert metallic gold to auf 5, metallic. Stable compounds of xenon form when. why can fluorine react with krypton to form a compound? krf 2 is an extremely strong oxidizing and fluorinating agent. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to. Krypton And Fluorine Reaction.
From pubs.rsc.org
The unique fluorine effects in organic reactions recent facts and Krypton And Fluorine Reaction why can fluorine react with krypton to form a compound? krf 2 is an extremely strong oxidizing and fluorinating agent. What colors are most pronounced in the. What is the electron configuration of krypton? in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. It can convert metallic gold. Krypton And Fluorine Reaction.
From www.chemistryscl.com
how to balance water and fluorine reaction Krypton And Fluorine Reaction Stable compounds of xenon form when. What colors are most pronounced in the. What is the electron configuration of krypton? krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. why can fluorine react with krypton to form a compound? It can convert metallic gold to auf 5, metallic. reaction of fluorine with noble. Krypton And Fluorine Reaction.
From www.researchgate.net
(PDF) Reaction of Fluorine Atoms with SiO2 Krypton And Fluorine Reaction krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. What is the electron configuration of krypton? reaction of fluorine with noble gasses. krf 2 is an extremely strong oxidizing and fluorinating agent. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. It can. Krypton And Fluorine Reaction.
From www.numerade.com
SOLVED This question is about compounds containing fluorine. (a) Draw Krypton And Fluorine Reaction krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. reaction of fluorine with noble gasses. krf 2 is an extremely strong oxidizing and fluorinating agent. Stable compounds of xenon form when. It can convert metallic gold to auf 5, metallic. What colors are most pronounced in the. What is the electron configuration of. Krypton And Fluorine Reaction.
From www.numerade.com
SOLVEDWrite a balanced equation for (a) the reaction between fluorine Krypton And Fluorine Reaction in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. What is the electron configuration of krypton? reaction of fluorine with noble gasses. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. Stable compounds of xenon form when. why can fluorine react with krypton. Krypton And Fluorine Reaction.
From www.vedantu.com
How is fluorine prepared by WhytlawGray method? Draw a diagram. Krypton And Fluorine Reaction krf 2 is an extremely strong oxidizing and fluorinating agent. why can fluorine react with krypton to form a compound? krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. What colors are most pronounced in the. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form. Krypton And Fluorine Reaction.
From www.researchgate.net
Glyphosate reactions with Fluorine, HF or Fluoride ion? Krypton And Fluorine Reaction What is the electron configuration of krypton? krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. reaction of fluorine with noble gasses. What colors are most pronounced in the. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. krf 2 is an extremely. Krypton And Fluorine Reaction.
From www.researchgate.net
(PDF) Highenergy krypton fluoride lasers for inertial fusion Krypton And Fluorine Reaction why can fluorine react with krypton to form a compound? in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. Stable compounds of xenon form when. What colors are most pronounced in the. krf 2 is an extremely strong oxidizing and fluorinating agent. What is the electron configuration of. Krypton And Fluorine Reaction.
From www.researchgate.net
Key components of a krypton fluoride (KrF) laser. Download Scientific Krypton And Fluorine Reaction It can convert metallic gold to auf 5, metallic. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. krf 2 is an extremely strong oxidizing and fluorinating agent. What is the electron configuration of krypton? why can fluorine react with krypton to form a compound? krypton forms. Krypton And Fluorine Reaction.
From pubs.acs.org
Fluorination of an Alumina Surface Modeling AluminumFluorine Reaction Krypton And Fluorine Reaction krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. krf 2 is an extremely strong oxidizing and fluorinating agent. why can fluorine react with krypton to form a compound? What is the electron configuration of krypton? in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form. Krypton And Fluorine Reaction.
From ar.inspiredpencil.com
Fluorine Molecular Structure Krypton And Fluorine Reaction krf 2 is an extremely strong oxidizing and fluorinating agent. What colors are most pronounced in the. Stable compounds of xenon form when. why can fluorine react with krypton to form a compound? It can convert metallic gold to auf 5, metallic. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to. Krypton And Fluorine Reaction.
From pediaa.com
Difference Between Fluorine and Fluoride Definition, Properties Krypton And Fluorine Reaction why can fluorine react with krypton to form a compound? What colors are most pronounced in the. It can convert metallic gold to auf 5, metallic. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta.. Krypton And Fluorine Reaction.
From www.photonicsonline.com
How One Light Source Manufacturer Overcame The 2015 Neon Crisis Krypton And Fluorine Reaction krf 2 is an extremely strong oxidizing and fluorinating agent. What colors are most pronounced in the. Stable compounds of xenon form when. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. It can convert metallic gold to auf 5, metallic. What is the electron configuration of krypton? . Krypton And Fluorine Reaction.
From chemistry-europe.onlinelibrary.wiley.com
Phosphine‐Catalyzed Acyl‐Group Exchange Reaction of Carboxylic Acids Krypton And Fluorine Reaction reaction of fluorine with noble gasses. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. It can convert metallic gold to auf 5, metallic. What is the electron configuration of krypton? why can fluorine react with krypton to form a compound? in each method, the highly reactive fluorine radicals react with krypton. Krypton And Fluorine Reaction.
From pubs.rsc.org
The unique fluorine effects in organic reactions recent facts and Krypton And Fluorine Reaction reaction of fluorine with noble gasses. What colors are most pronounced in the. krf 2 is an extremely strong oxidizing and fluorinating agent. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. It can. Krypton And Fluorine Reaction.
From www.mdpi.com
Pharmaceuticals Free FullText Fluorinated Protein and Peptide Krypton And Fluorine Reaction why can fluorine react with krypton to form a compound? Stable compounds of xenon form when. What colors are most pronounced in the. krf 2 is an extremely strong oxidizing and fluorinating agent. It can convert metallic gold to auf 5, metallic. What is the electron configuration of krypton? reaction of fluorine with noble gasses. krypton. Krypton And Fluorine Reaction.
From www.researchgate.net
Strategies for the assembly of sulfonyl fluorides Download Scientific Krypton And Fluorine Reaction Stable compounds of xenon form when. why can fluorine react with krypton to form a compound? reaction of fluorine with noble gasses. in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. It can convert metallic gold to auf 5, metallic. What colors are most pronounced in the. . Krypton And Fluorine Reaction.
From thechemistrynotes.com
Krypton (Kr) Element Properties, Interesting Uses, Facts Krypton And Fluorine Reaction in each method, the highly reactive fluorine radicals react with krypton at low temperatures to form the meta. Stable compounds of xenon form when. why can fluorine react with krypton to form a compound? What colors are most pronounced in the. krf 2 is an extremely strong oxidizing and fluorinating agent. krypton forms a difluoride, krf. Krypton And Fluorine Reaction.