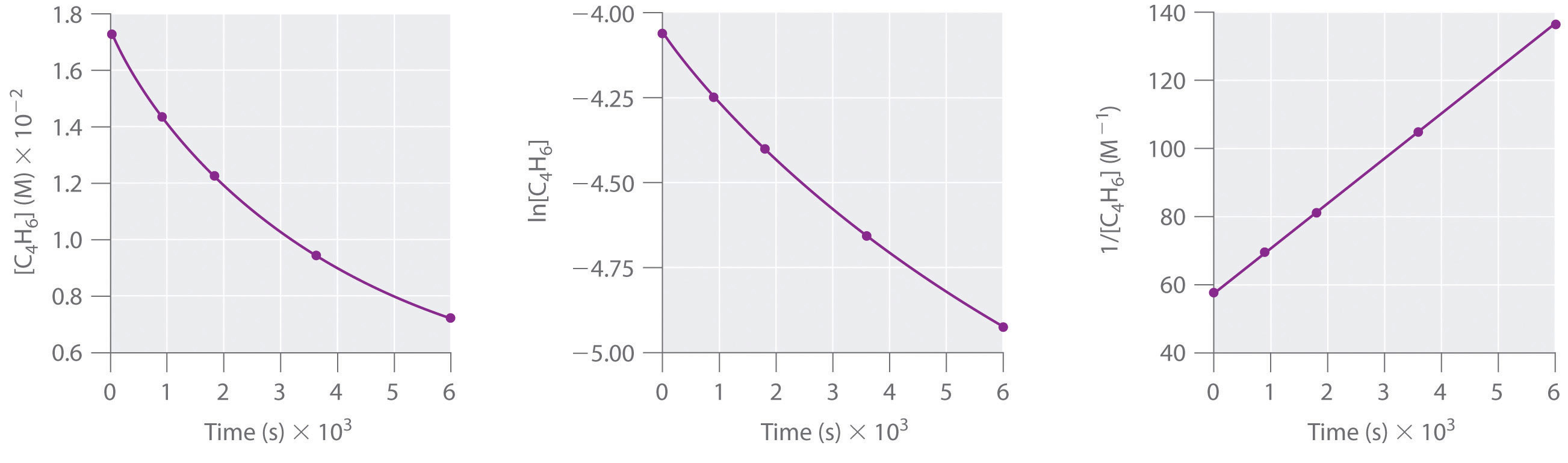
The Unit Rate Constant For First Order Reaction Is . The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. However, the units of k vary. For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of the reactants is falling. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. D [a]/dt denotes the change in the concentration of.
from chem.libretexts.org
The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. D [a]/dt denotes the change in the concentration of. For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of the reactants is falling. However, the units of k vary.
Chapter 13.4 Using Graphs to Determine Rate Laws, Rate Constants and
The Unit Rate Constant For First Order Reaction Is The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. D [a]/dt denotes the change in the concentration of. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. However, the units of k vary. For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of the reactants is falling. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time).
From mavink.com
Rate Constant Calculation The Unit Rate Constant For First Order Reaction Is The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. However, the units of k vary. More generally speaking, the units for the rate. The Unit Rate Constant For First Order Reaction Is.
From byjus.com
What is the unit of rate constant for first order reaction The Unit Rate Constant For First Order Reaction Is The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. D [a]/dt denotes the change in the concentration of. For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one. The Unit Rate Constant For First Order Reaction Is.
From www.slideserve.com
PPT SCH4U Unit 1 Energy Changes & Rates of Reactions (Cont’d The Unit Rate Constant For First Order Reaction Is The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. The rate constant, k, is a proportionality constant that. The Unit Rate Constant For First Order Reaction Is.
From www.tessshebaylo.com
Derive An Integrated Rate Equation For Constant The First Order The Unit Rate Constant For First Order Reaction Is However, the units of k vary. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. D [a]/dt denotes. The Unit Rate Constant For First Order Reaction Is.
From mungfali.com
The Rate Constant For Zero Order Reaction Is Youtube EF4 The Unit Rate Constant For First Order Reaction Is However, the units of k vary. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the. The Unit Rate Constant For First Order Reaction Is.
From www.slideserve.com
PPT Reaction 1 st order reactions PowerPoint Presentation The Unit Rate Constant For First Order Reaction Is The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. For the purposes of rate. The Unit Rate Constant For First Order Reaction Is.
From chem.libretexts.org
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and The Unit Rate Constant For First Order Reaction Is The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). However, the units of k vary. More generally speaking, the units for the rate. The Unit Rate Constant For First Order Reaction Is.
From general.chemistrysteps.com
HalfLife of a Reaction Chemistry Steps The Unit Rate Constant For First Order Reaction Is The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. For the purposes of rate. The Unit Rate Constant For First Order Reaction Is.
From www.toppr.com
Units of rate constant of a first order reaction is The Unit Rate Constant For First Order Reaction Is However, the units of k vary. D [a]/dt denotes the change in the concentration of. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of. The Unit Rate Constant For First Order Reaction Is.
From general.chemistrysteps.com
Rate Law and Reaction Order Chemistry Steps The Unit Rate Constant For First Order Reaction Is However, the units of k vary. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. D [a]/dt denotes the change in the concentration of. For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of. The Unit Rate Constant For First Order Reaction Is.
From www.tessshebaylo.com
Derive Integrated Rate Equation For First Order Reaction Tessshebaylo The Unit Rate Constant For First Order Reaction Is The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. D [a]/dt denotes the change in the concentration of. The rate constant k and. The Unit Rate Constant For First Order Reaction Is.
From byjus.com
The rate constant of a reaction is 1.2×10^ 3sec^ 1 at 30℃and 2.1×10 The Unit Rate Constant For First Order Reaction Is D [a]/dt denotes the change in the concentration of. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. However, the units of k vary. The rate. The Unit Rate Constant For First Order Reaction Is.
From chemguru.sg
Rate Equation and Order of Reaction The Unit Rate Constant For First Order Reaction Is However, the units of k vary. D [a]/dt denotes the change in the concentration of. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. The rate constant, k, is a proportionality constant that indicates the relationship between the molar. The Unit Rate Constant For First Order Reaction Is.
From askfilo.com
Units of rate constant for a first order reaction. Filo The Unit Rate Constant For First Order Reaction Is More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. The rate is the reaction rate (in units of molar/time) and k. The Unit Rate Constant For First Order Reaction Is.
From general.chemistrysteps.com
Determining Reaction Order Using Graphs Chemistry Steps The Unit Rate Constant For First Order Reaction Is More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate. The Unit Rate Constant For First Order Reaction Is.
From giozvfsai.blob.core.windows.net
The Rate Constant For 1St Order Reaction Is 69.3 at Jacklyn Bowman blog The Unit Rate Constant For First Order Reaction Is The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. D [a]/dt denotes the change in the concentration of. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate. The Unit Rate Constant For First Order Reaction Is.
From www.slideserve.com
PPT Summary of the of ZeroOrder, FirstOrder and Second The Unit Rate Constant For First Order Reaction Is The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. For the purposes of rate equations and orders of reaction, the rate of a reaction is measured. The Unit Rate Constant For First Order Reaction Is.
From cealqzpu.blob.core.windows.net
Unit Of Velocity Constant For Zero Order Reaction at Larry Hetzel blog The Unit Rate Constant For First Order Reaction Is However, the units of k vary. D [a]/dt denotes the change in the concentration of. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. For the purposes of rate equations. The Unit Rate Constant For First Order Reaction Is.
From www.youtube.com
Units of k for Zero, 1st, and 2nd Order Reactions YouTube The Unit Rate Constant For First Order Reaction Is For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of the reactants is falling. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). More generally speaking, the units for the rate. The Unit Rate Constant For First Order Reaction Is.
From www.youtube.com
Integrated Rate Equation for third Order Reaction Third Order The Unit Rate Constant For First Order Reaction Is The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. D [a]/dt denotes the change in the concentration of. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. The rate is the reaction rate (in units. The Unit Rate Constant For First Order Reaction Is.
From mungfali.com
The Rate Constant For Zero Order Reaction Is Youtube EF4 The Unit Rate Constant For First Order Reaction Is D [a]/dt denotes the change in the concentration of. However, the units of k vary. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. The rate constant, k, is a proportionality constant that indicates the relationship between the molar. The Unit Rate Constant For First Order Reaction Is.
From www.slideserve.com
PPT Chapter 14 Chemical PowerPoint Presentation, free The Unit Rate Constant For First Order Reaction Is The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of the reactants is. The Unit Rate Constant For First Order Reaction Is.
From www.chem.fsu.edu
The Unit Rate Constant For First Order Reaction Is The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. D [a]/dt denotes the change in the concentration of. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. For the purposes. The Unit Rate Constant For First Order Reaction Is.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions The Unit Rate Constant For First Order Reaction Is More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). D [a]/dt denotes the change in the concentration of. However, the units of k vary. The rate constant, k, is a. The Unit Rate Constant For First Order Reaction Is.
From www.youtube.com
Units of rate constant YouTube The Unit Rate Constant For First Order Reaction Is For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of the reactants is falling. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). D [a]/dt denotes the change in the concentration. The Unit Rate Constant For First Order Reaction Is.
From www.tpsearchtool.com
How To Calculate The Order Of A Reaction And The Rate Constant Youtube The Unit Rate Constant For First Order Reaction Is More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants. For the purposes of rate equations and orders of reaction, the rate. The Unit Rate Constant For First Order Reaction Is.
From chemistryguru.com.sg
Rate Equation and Order of Reaction The Unit Rate Constant For First Order Reaction Is The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). For the purposes of rate equations and orders of reaction, the rate of a. The Unit Rate Constant For First Order Reaction Is.
From www.youtube.com
PCAT Units for Rate Constant Zeroth Order, First Order, Second Order The Unit Rate Constant For First Order Reaction Is However, the units of k vary. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. More generally speaking, the units for the rate. The Unit Rate Constant For First Order Reaction Is.
From www.toppr.com
The unit of rate constant for a zero order reaction is The Unit Rate Constant For First Order Reaction Is The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of the reactants is falling. D [a]/dt denotes the change in the concentration. The Unit Rate Constant For First Order Reaction Is.
From general.chemistrysteps.com
Determining Reaction Order Using Graphs Chemistry Steps The Unit Rate Constant For First Order Reaction Is The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). However, the units of k vary. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. The rate constant, k, is a proportionality constant that indicates the relationship between the molar. The Unit Rate Constant For First Order Reaction Is.
From chem.libretexts.org
Chapter 13.4 Using Graphs to Determine Rate Laws, Rate Constants and The Unit Rate Constant For First Order Reaction Is The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration of one of the reactants is falling. However, the units of. The Unit Rate Constant For First Order Reaction Is.
From chemistry.stackexchange.com
physical chemistry Formula for rate constant for the first order The Unit Rate Constant For First Order Reaction Is The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. For the purposes of rate equations and orders of reaction, the rate of a. The Unit Rate Constant For First Order Reaction Is.
From www.reddit.com
[Chemical Engineering] What topics to revise for these chemical The Unit Rate Constant For First Order Reaction Is D [a]/dt denotes the change in the concentration of. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). However, the units of k vary. The rate constant k and the. The Unit Rate Constant For First Order Reaction Is.
From www.youtube.com
Units of rate constant calculate rate constant units units of first The Unit Rate Constant For First Order Reaction Is The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce. For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how. The Unit Rate Constant For First Order Reaction Is.
From www.slideshare.net
Lect w2 152 rate laws_alg The Unit Rate Constant For First Order Reaction Is D [a]/dt denotes the change in the concentration of. However, the units of k vary. The rate is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time). For the purposes of rate equations and orders of reaction, the rate of a reaction is measured in terms of how fast the concentration. The Unit Rate Constant For First Order Reaction Is.