Buffers Used In Biological Systems . the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers in biology and biological buffers. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. you should be aware that buffers play a critical role in almost all biochemical systems. In this way, a biological buffer. the use of buffers that mimic biological solutions is a foundation of biochemical studies. Biochemical experiments routinely require a. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). One of the most common buffering. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions.
from www.youtube.com
In this way, a biological buffer. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. buffers in biology and biological buffers. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. One of the most common buffering. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be aware that buffers play a critical role in almost all biochemical systems. the use of buffers that mimic biological solutions is a foundation of biochemical studies. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time).
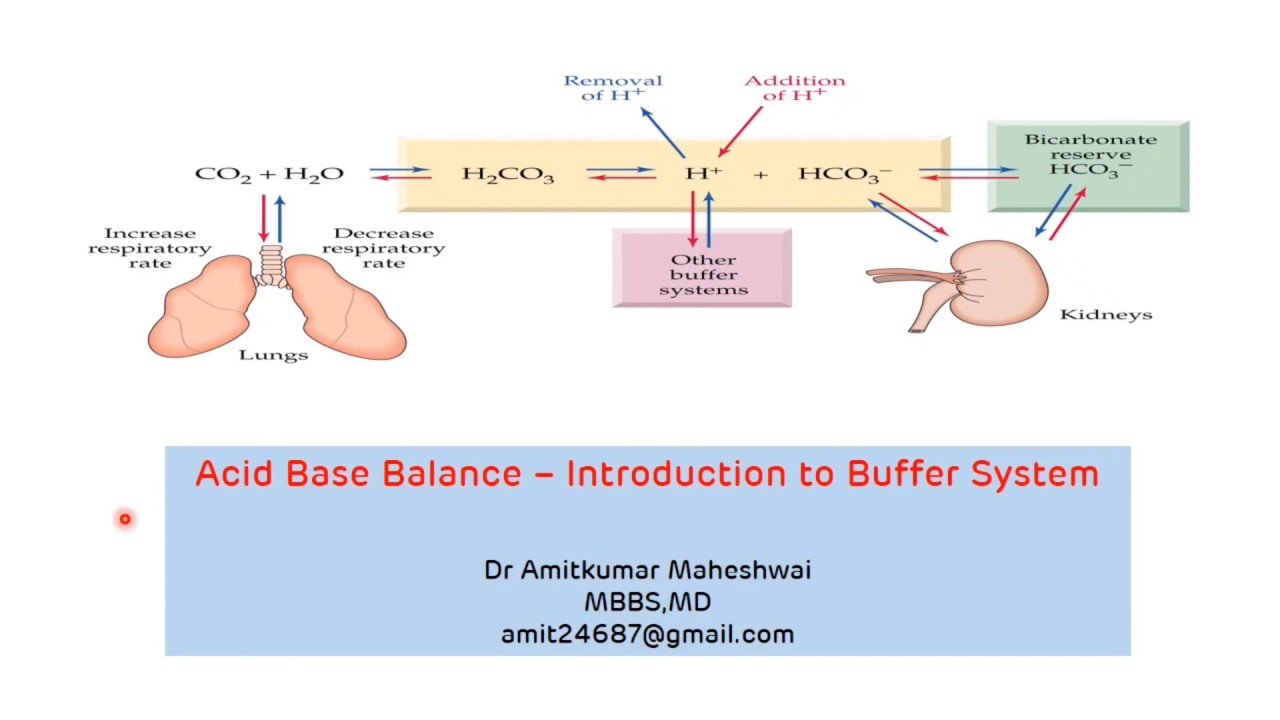
Introduction to Buffer System Regulation of pH Acid Base Balance
Buffers Used In Biological Systems you should be aware that buffers play a critical role in almost all biochemical systems. the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical role in almost all biochemical systems. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. One of the most common buffering. buffers in biology and biological buffers. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). In this way, a biological buffer. Biochemical experiments routinely require a.
From exoxulnnf.blob.core.windows.net
Biological Roles Of Buffers at Dana Wild blog Buffers Used In Biological Systems the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. One of the most common buffering. Biochemical experiments routinely require a. buffers in biology and biological buffers. In this way, a biological buffer. the purpose. Buffers Used In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Buffers Used In Biological Systems Biochemical experiments routinely require a. In this way, a biological buffer. One of the most common buffering. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. you should be aware that buffers play a critical. Buffers Used In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Buffers Used In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist.. Buffers Used In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffers Used In Biological Systems Biochemical experiments routinely require a. buffers in biology and biological buffers. you should be aware that buffers play a critical role in almost all biochemical systems. the use of buffers that mimic biological solutions is a foundation of biochemical studies. In this way, a biological buffer. the most relevant systems for biology are the carbonic acid/carbonate. Buffers Used In Biological Systems.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free Buffers Used In Biological Systems In this way, a biological buffer. you should be aware that buffers play a critical role in almost all biochemical systems. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. the use of buffers that mimic biological solutions is a foundation of biochemical. Buffers Used In Biological Systems.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Buffers Used In Biological Systems the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical role in almost all biochemical systems. the most relevant systems. Buffers Used In Biological Systems.
From exoxnpydf.blob.core.windows.net
Biological Buffer Chart at Megan Hankins blog Buffers Used In Biological Systems buffers in biology and biological buffers. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the purpose of a buffer in a biological system is to maintain intracellular and. Buffers Used In Biological Systems.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Buffers Used In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). the purpose of a. Buffers Used In Biological Systems.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffers Used In Biological Systems the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). buffers in biology and biological. Buffers Used In Biological Systems.
From slideplayer.com
Chapter 2 Chemistry ppt download Buffers Used In Biological Systems you should be aware that buffers play a critical role in almost all biochemical systems. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. One of the most common buffering. buffers in biology and biological buffers. the use of buffers that mimic biological solutions is a foundation of biochemical. Buffers Used In Biological Systems.
From www.slideshare.net
Buffers in biological systems Buffers Used In Biological Systems Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range. Buffers Used In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Buffers Used In Biological Systems Biochemical experiments routinely require a. One of the most common buffering. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). you should be aware that buffers play a critical role. Buffers Used In Biological Systems.
From exoxulnnf.blob.core.windows.net
Biological Roles Of Buffers at Dana Wild blog Buffers Used In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biochemical experiments routinely require a. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very. Buffers Used In Biological Systems.
From www.youtube.com
Buffer in biological system buffer system in blood buffer in eyes Buffers Used In Biological Systems you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. One of. Buffers Used In Biological Systems.
From www.researchgate.net
(PDF) Buffers for Use in Biological Systems Buffers Used In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Biochemical experiments routinely require a. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and. Buffers Used In Biological Systems.
From ceaaclpe.blob.core.windows.net
Buffer In Biology Example at Richard Durbin blog Buffers Used In Biological Systems the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers in biology and biological buffers. you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One of. Buffers Used In Biological Systems.
From www.tffn.net
What is a Buffer in Science? Exploring the Role of Buffers in Chemistry Buffers Used In Biological Systems the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be aware that buffers play a critical role in almost all biochemical systems. the use of buffers that mimic biological solutions. Buffers Used In Biological Systems.
From www.pinterest.com
What are the Buffers and its Importance? This article explains the Buffers Used In Biological Systems a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biochemical experiments routinely require a. you should be aware that buffers play a critical role in almost all biochemical systems. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). In this. Buffers Used In Biological Systems.
From www.youtube.com
buffer isotonic solution buffer capacity buffers in pharmaceutical Buffers Used In Biological Systems Biochemical experiments routinely require a. In this way, a biological buffer. the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical role in almost all biochemical systems. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological systems. Buffers Used In Biological Systems.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Buffers Used In Biological Systems Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this way, a biological buffer. you should be aware that buffers play a critical role in almost all biochemical. Buffers Used In Biological Systems.
From dxoemziow.blob.core.windows.net
Buffers Biological Research at Agustin Negrete blog Buffers Used In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. One of the most common buffering. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls. Buffers Used In Biological Systems.
From ceaaclpe.blob.core.windows.net
Buffer In Biology Example at Richard Durbin blog Buffers Used In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). you should be aware that. Buffers Used In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Buffers Used In Biological Systems the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. Biochemical experiments routinely require a. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood. Buffers Used In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffers Used In Biological Systems Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). the use of buffers that mimic biological solutions is a foundation of biochemical studies. In this way, a biological buffer. you should be aware that buffers play a critical role in almost all biochemical systems. Biochemical experiments. Buffers Used In Biological Systems.
From www.youtube.com
Buffer Capacity 03 Buffers in pharmaceutical and biological systems Buffers Used In Biological Systems the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. Biochemical experiments routinely require a. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). the use of buffers that mimic biological solutions is. Buffers Used In Biological Systems.
From www.protocols.io
Buffers for Use in Biological Systems Buffers Used In Biological Systems One of the most common buffering. In this way, a biological buffer. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biochemical experiments routinely require a. the use. Buffers Used In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffers Used In Biological Systems a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. Biochemical experiments routinely require a. the use of buffers that mimic biological solutions is a foundation of biochemical studies. Biological systems have peak activity in a. Buffers Used In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Buffers Used In Biological Systems the use of buffers that mimic biological solutions is a foundation of biochemical studies. Biochemical experiments routinely require a. you should be aware that buffers play a critical role in almost all biochemical systems. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. In this way, a biological buffer. . Buffers Used In Biological Systems.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Buffers Used In Biological Systems One of the most common buffering. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). buffers in biology and biological buffers. In this way, a biological buffer. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. you should. Buffers Used In Biological Systems.
From www.slideserve.com
PPT UNIT V pH, BUFFERS AND ISOTONIC SOLUTION PowerPoint Presentation Buffers Used In Biological Systems you should be aware that buffers play a critical role in almost all biochemical systems. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. In this way, a biological buffer.. Buffers Used In Biological Systems.
From www.slideshare.net
Buffers in biological systems Buffers Used In Biological Systems you should be aware that buffers play a critical role in almost all biochemical systems. buffers in biology and biological buffers. Biochemical experiments routinely require a. the use of buffers that mimic biological solutions is a foundation of biochemical studies. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph. Buffers Used In Biological Systems.
From www.youtube.com
CHEM2114 Lecture 13 Buffers in the Biological Systems YouTube Buffers Used In Biological Systems One of the most common buffering. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. buffers in biology and biological buffers. the use of buffers that mimic biological solutions is a foundation of biochemical studies. you should be aware that buffers play a critical role in almost all biochemical. Buffers Used In Biological Systems.
From www.youtube.com
Biological Buffer Systems Role of Buffers in biological system Buffers Used In Biological Systems In this way, a biological buffer. the use of buffers that mimic biological solutions is a foundation of biochemical studies. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biochemical experiments routinely require a. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. . Buffers Used In Biological Systems.
From www.youtube.com
Role of buffers in biological system YouTube Buffers Used In Biological Systems the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. the use of buffers that mimic biological solutions is a foundation of biochemical studies. a biological. Buffers Used In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Buffers Used In Biological Systems Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). One of the most common buffering. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. the purpose of a buffer in a biological system is to maintain intracellular and extracellular. Buffers Used In Biological Systems.