Catalyst Meaning Function . They do this by lowering the energy used when proceeding from reactants to products in a. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts by formal definition are substances designed to increase the rate of a reaction. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is some material that speeds up chemical reactions. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions.
from medicaltaste.weebly.com
The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysts by formal definition are substances designed to increase the rate of a reaction. They do this by lowering the energy used when proceeding from reactants to products in a. A catalyst is some material that speeds up chemical reactions.
Periodic table catalyst definition chemistry medicaltaste
Catalyst Meaning Function Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts by formal definition are substances designed to increase the rate of a reaction. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. They do this by lowering the energy used when proceeding from reactants to products in a. A catalyst is some material that speeds up chemical reactions. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but.
From www.youtube.com
Definition of Catalyst Chemical Chemistry Class 12 YouTube Catalyst Meaning Function A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is a chemical substance that affects the. Catalyst Meaning Function.
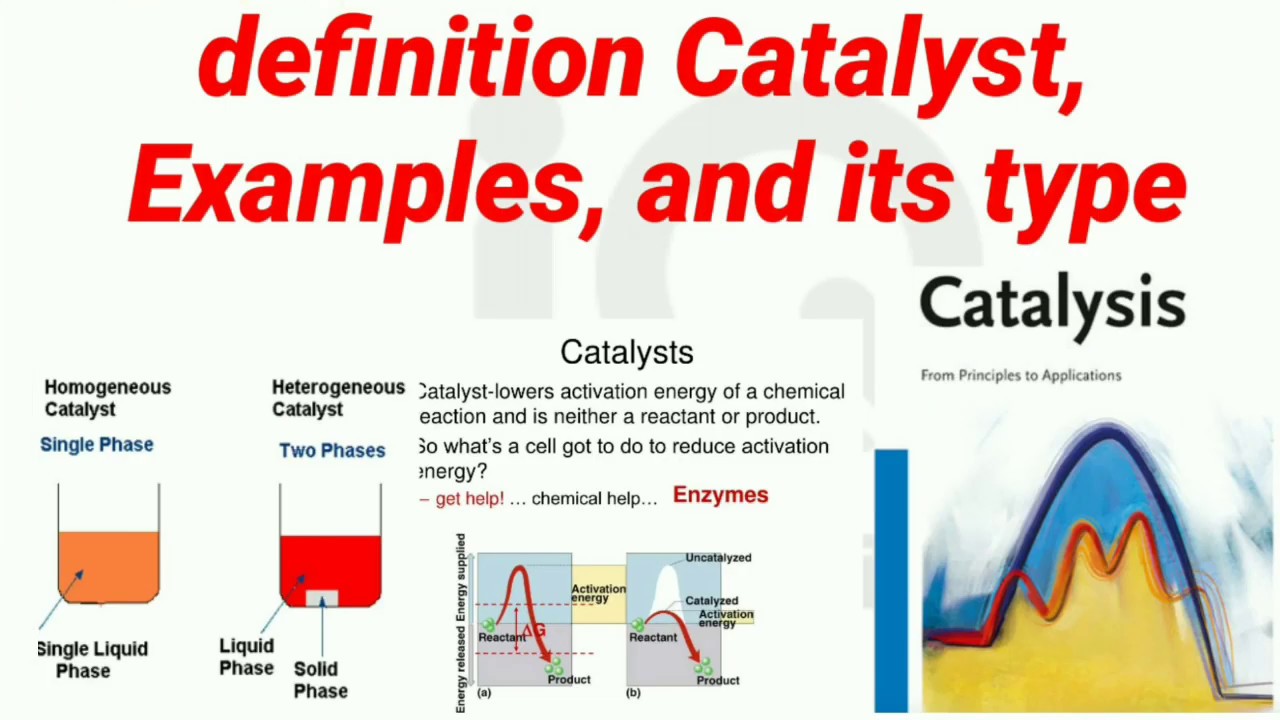
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2380033 Catalyst Meaning Function Catalysts by formal definition are substances designed to increase the rate of a reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst, in turn, is a substance that is not consumed by the chemical reaction,. Catalyst Meaning Function.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst Meaning Function The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A. Catalyst Meaning Function.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Meaning Function Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. The meaning of. Catalyst Meaning Function.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID4752647 Catalyst Meaning Function A catalyst is some material that speeds up chemical reactions. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a chemical substance that affects the rate of a chemical reaction. Catalyst Meaning Function.
From www.slideshare.net
Catalyst Catalyst Meaning Function A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. Catalysts by formal. Catalyst Meaning Function.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID4503154 Catalyst Meaning Function They do this by lowering the energy used when proceeding from reactants to products in a. Catalysts by formal definition are substances designed to increase the rate of a reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is defined as increasing the rate of a chemical reaction by introducing a. Catalyst Meaning Function.
From www.researchgate.net
Classification of catalyst. Download Scientific Diagram Catalyst Meaning Function A catalyst is some material that speeds up chemical reactions. Catalysts by formal definition are substances designed to increase the rate of a reaction. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a. Catalyst Meaning Function.
From www.pinterest.com
Meaning of catalyst English vocabulary words, Vocabulary words, English vocabulary Catalyst Meaning Function The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysis is defined as increasing. Catalyst Meaning Function.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Presentation ID1988156 Catalyst Meaning Function Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. The meaning of catalyst. Catalyst Meaning Function.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Meaning Function Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. A catalyst is a chemical. Catalyst Meaning Function.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free download ID2683753 Catalyst Meaning Function With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. They do this by lowering the energy used when proceeding from reactants to products in a. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. A catalyst is some material that speeds up. Catalyst Meaning Function.
From www.slideserve.com
PPT Transportation PowerPoint Presentation, free download ID969043 Catalyst Meaning Function A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do this by lowering the energy used when proceeding from reactants to products in a. A catalyst is some material that speeds up chemical reactions. Catalysts by. Catalyst Meaning Function.
From www.slideserve.com
PPT CATALYSIS PowerPoint Presentation, free download ID270211 Catalyst Meaning Function They do this by lowering the energy used when proceeding from reactants to products in a. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysts by formal definition are substances designed to increase the rate of a reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself. Catalyst Meaning Function.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst. Download Scientific Catalyst Meaning Function Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. They do this by lowering the energy used when proceeding from reactants to products in a. Catalysts by formal. Catalyst Meaning Function.
From gamesmartz.com
Catalyst Definition & Image GameSmartz Catalyst Meaning Function Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. A catalyst is some material that speeds up chemical reactions. A catalyst is a chemical substance that affects the rate of a. Catalyst Meaning Function.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between homogeneous and heterogeneous Catalyst Meaning Function Catalysts by formal definition are substances designed to increase the rate of a reaction. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst is some material that speeds up chemical reactions. The meaning of catalyst is. Catalyst Meaning Function.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation, free download ID5736986 Catalyst Meaning Function Catalysts by formal definition are substances designed to increase the rate of a reaction. A catalyst is some material that speeds up chemical reactions. They do this by lowering the energy used when proceeding from reactants to products in a. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or. Catalyst Meaning Function.
From study.com
Catalyst Definition, Types & Function Lesson Catalyst Meaning Function They do this by lowering the energy used when proceeding from reactants to products in a. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate. Catalyst Meaning Function.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between homogeneous and heterogeneous Catalyst Meaning Function They do this by lowering the energy used when proceeding from reactants to products in a. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. A catalyst is some material that speeds up chemical reactions. Catalysts by formal definition are substances designed to increase the rate of a reaction. Catalysis is defined as. Catalyst Meaning Function.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of bind, matter 218305638 Catalyst Meaning Function Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. They do this by lowering the energy used when proceeding from reactants to products in a. With a. Catalyst Meaning Function.
From www.youtube.com
Identifying function of catalyst in a reaction YouTube Catalyst Meaning Function They do this by lowering the energy used when proceeding from reactants to products in a. Catalysts by formal definition are substances designed to increase the rate of a reaction. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. With a helping hand from a catalyst,. Catalyst Meaning Function.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Presentation ID1988156 Catalyst Meaning Function Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysts by formal definition are substances designed to increase. Catalyst Meaning Function.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free download ID6634764 Catalyst Meaning Function The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the. Catalyst Meaning Function.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalyst Meaning Function With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts by formal definition are substances designed to increase the rate of a reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is defined as increasing the rate of a chemical. Catalyst Meaning Function.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between homogeneous and heterogeneous Catalyst Meaning Function With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. The meaning of catalyst is a substance that enables a chemical reaction to proceed at. Catalyst Meaning Function.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of catalyst [Animation Video Catalyst Meaning Function They do this by lowering the energy used when proceeding from reactants to products in a. A catalyst is some material that speeds up chemical reactions. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts by formal definition are substances designed to increase. Catalyst Meaning Function.
From www.slideserve.com
PPT Nanocatalyst PowerPoint Presentation ID676158 Catalyst Meaning Function The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. Catalysts by formal definition are substances designed to increase the rate of a reaction. Catalysis is defined as increasing the rate of a chemical reaction by introducing a catalyst. A catalyst, in turn, is a substance that. Catalyst Meaning Function.
From www.youtube.com
What Does A Catalyst Do? YouTube Catalyst Meaning Function Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. A catalyst is some material that speeds up chemical reactions. Catalysts by formal definition are substances designed to increase the rate. Catalyst Meaning Function.
From www.slideserve.com
PPT Lesson Five PowerPoint Presentation ID2215771 Catalyst Meaning Function They do this by lowering the energy used when proceeding from reactants to products in a. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, in. Catalyst Meaning Function.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Meaning Function Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for. Catalyst Meaning Function.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Meaning Function A catalyst, in turn, is a substance that is not consumed by the chemical reaction, but. Catalysts by formal definition are substances designed to increase the rate of a reaction. They do this by lowering the energy used when proceeding from reactants to products in a. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself. Catalyst Meaning Function.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between homogeneous and heterogeneous Catalyst Meaning Function Catalysts by formal definition are substances designed to increase the rate of a reaction. They do this by lowering the energy used when proceeding from reactants to products in a. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to interact. Catalyst Meaning Function.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B"... Download Scientific Diagram Catalyst Meaning Function A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst, in turn, is a substance that is not consumed by the chemical reaction,. Catalyst Meaning Function.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Meaning Function A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. The meaning of catalyst is a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions. Catalysts by formal definition are substances designed to increase the rate of. Catalyst Meaning Function.