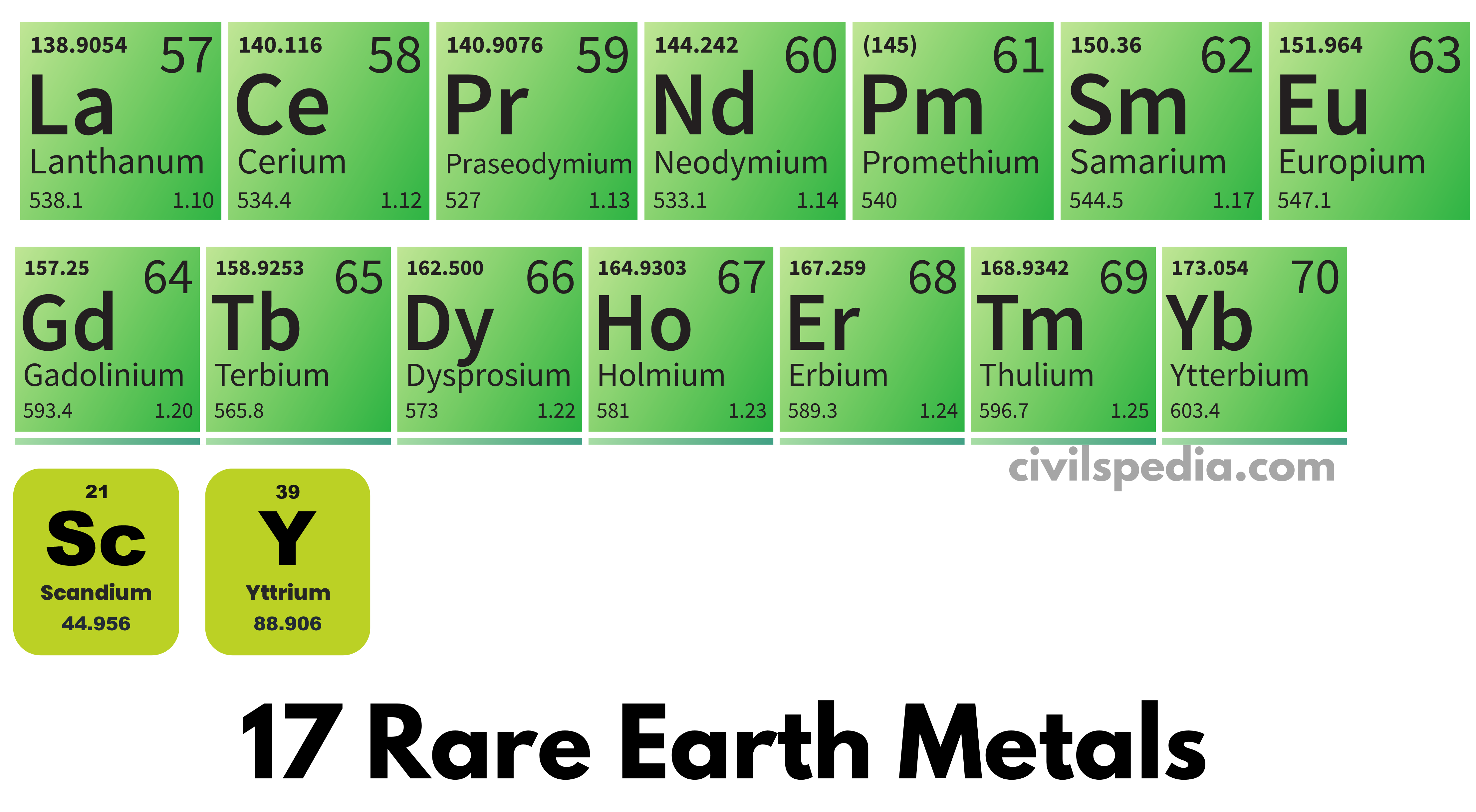
Why Is It Called Rare Earth Metals . The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. It’s significant that there are 15 rare earth elements: Rare earth elements are a collection of 17 elements on the. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. But what exactly are ‘rare earth’ elements and just how rare are they?
from civilspedia.com
It’s significant that there are 15 rare earth elements: The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. But what exactly are ‘rare earth’ elements and just how rare are they? Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. Rare earth elements are a collection of 17 elements on the. The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each.
Rare Earth Metals
Why Is It Called Rare Earth Metals The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. Rare earth elements are a collection of 17 elements on the. But what exactly are ‘rare earth’ elements and just how rare are they? Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. It’s significant that there are 15 rare earth elements: The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each.
From www.priyamstudycentre.com
Rare Earth Elements Metals, Definition, Properties, Uses Why Is It Called Rare Earth Metals Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. It’s significant that there are 15 rare earth elements: But what exactly are ‘rare earth’ elements and just how rare are they? The rare earth elements are a group of transition metals, found mainly in the first row below. Why Is It Called Rare Earth Metals.
From staging4.aicorespot.io
Interesting Facts About Rare Earth Metals AICorespot Why Is It Called Rare Earth Metals But what exactly are ‘rare earth’ elements and just how rare are they? Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. Rare earth elements are a collection of 17 elements on the. The rare earth elements are a group of transition metals, found mainly in the first. Why Is It Called Rare Earth Metals.
From www.globalxetfs.com
Rare Earth Elements, Explained Global X ETFs Why Is It Called Rare Earth Metals The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. But what exactly are ‘rare earth’ elements and just how rare are they? It’s significant that there are 15 rare earth elements: Rare earth elements are a collection of 17 elements on the. The rare earth elements are a group of. Why Is It Called Rare Earth Metals.
From www.cummins.com
What are tech metals and rare earth elements, and how are they used Why Is It Called Rare Earth Metals But what exactly are ‘rare earth’ elements and just how rare are they? Rare earth elements are a collection of 17 elements on the. It’s significant that there are 15 rare earth elements: The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. The rare earth elements are a group of. Why Is It Called Rare Earth Metals.
From carbonacea.blogspot.com
Carbonacea Rare earth elements in coal fly ash a possible resource? Presentation at 2015 GSA Why Is It Called Rare Earth Metals But what exactly are ‘rare earth’ elements and just how rare are they? The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. It’s significant that there are 15 rare earth elements: Chemistry students may recall that when electrons are added to an. Why Is It Called Rare Earth Metals.
From www.thoughtco.com
Rare Earth Elements (Metals) List Why Is It Called Rare Earth Metals It’s significant that there are 15 rare earth elements: The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. But what exactly are ‘rare. Why Is It Called Rare Earth Metals.
From civilspedia.com
Rare Earth Metals Why Is It Called Rare Earth Metals Rare earth elements are a collection of 17 elements on the. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. The. Why Is It Called Rare Earth Metals.
From www.slideserve.com
PPT What are rare earth metals? PowerPoint Presentation ID7266291 Why Is It Called Rare Earth Metals Rare earth elements are a collection of 17 elements on the. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. But what exactly are ‘rare earth’ elements and just how rare are they? The rare earth metals naturally have three positive charges. Why Is It Called Rare Earth Metals.
From www.americanelements.com
Rare Earths AMERICAN ELEMENTS Why Is It Called Rare Earth Metals Rare earth elements are a collection of 17 elements on the. The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. But what exactly are ‘rare earth’ elements and just. Why Is It Called Rare Earth Metals.
From www.britannica.com
Rareearth element Properties, Metals, Uses Britannica Why Is It Called Rare Earth Metals It’s significant that there are 15 rare earth elements: The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. The rare earth. Why Is It Called Rare Earth Metals.
From www.livescience.com
Facts About Rare Earth Elements (Infographic) Live Science Why Is It Called Rare Earth Metals The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. It’s significant that there are 15 rare earth elements: But what exactly are ‘rare earth’ elements and just how rare are they? Chemistry students may recall that when electrons are added to an. Why Is It Called Rare Earth Metals.
From www.rareelementresources.com
Rare Earth Elements Why Is It Called Rare Earth Metals It’s significant that there are 15 rare earth elements: The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. Rare earth elements are a. Why Is It Called Rare Earth Metals.
From mavink.com
Rare Earth Metals On Periodic Table Why Is It Called Rare Earth Metals But what exactly are ‘rare earth’ elements and just how rare are they? It’s significant that there are 15 rare earth elements: Rare earth elements are a collection of 17 elements on the. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium.. Why Is It Called Rare Earth Metals.
From www.researchgate.net
Applications of rare earth metals (REMs). Download Scientific Diagram Why Is It Called Rare Earth Metals It’s significant that there are 15 rare earth elements: But what exactly are ‘rare earth’ elements and just how rare are they? Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. Rare earth elements are a collection of 17 elements on the. The rare earth elements are a. Why Is It Called Rare Earth Metals.
From www.jxscmachine.com
What's Rare Earth Elements Minerals, Uses JXSC Machine Why Is It Called Rare Earth Metals Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. Rare earth elements are a collection of 17 elements on the. The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. But what exactly are ‘rare earth’ elements and just. Why Is It Called Rare Earth Metals.
From www.gzwfen.com
Rare Earth Metals Scandium,Scandium Why Is It Called Rare Earth Metals The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. It’s significant that there are 15 rare earth elements: But what exactly are ‘rare earth’ elements and just how rare. Why Is It Called Rare Earth Metals.
From dy6metals.com
Heavy Rare Earth DY6 Why Is It Called Rare Earth Metals Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. Rare earth elements are a collection of 17 elements on the. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. The. Why Is It Called Rare Earth Metals.
From periodictablegroups.wordpress.com
Rare Earth Metals Periodic Table Groups Why Is It Called Rare Earth Metals It’s significant that there are 15 rare earth elements: The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. Rare earth elements are a. Why Is It Called Rare Earth Metals.
From www.studyiq.com
Rare Earth Metals Why Is It Called Rare Earth Metals Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. But what exactly are ‘rare earth’ elements and just how rare are they? Rare earth elements are a collection of 17 elements on the. The rare earth elements are a group of transition metals, found mainly in the first. Why Is It Called Rare Earth Metals.
From www.techmetalsresearch.com
What Are Rare Earth Metals? What Are They Used For? Why Is It Called Rare Earth Metals The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. Rare earth elements are a collection of 17 elements on the. The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. It’s significant that. Why Is It Called Rare Earth Metals.
From www.gzwfen.com
Rare Earth Metals Scandium,Scandium Why Is It Called Rare Earth Metals The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. Rare earth elements are a collection of 17 elements on the. But what exactly are ‘rare earth’ elements and just how rare are they? The rare earth metals naturally have three positive charges. Why Is It Called Rare Earth Metals.
From newseu.cgtn.com
What are rare earth metals and what are they used for? CGTN Why Is It Called Rare Earth Metals But what exactly are ‘rare earth’ elements and just how rare are they? Rare earth elements are a collection of 17 elements on the. The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. It’s significant that there are 15 rare earth elements: Chemistry students may recall that when electrons are. Why Is It Called Rare Earth Metals.
From unbelievable-facts.com
What are Rare Earth Elements, and why are they so named? Why Is It Called Rare Earth Metals Rare earth elements are a collection of 17 elements on the. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. The. Why Is It Called Rare Earth Metals.
From www.stanfordmaterials.com
What is Rareearth Element? How is it Used? Why Is It Called Rare Earth Metals Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. But what exactly are ‘rare earth’ elements and just how rare are they? It’s significant that there are 15 rare earth elements: Rare earth elements are a collection of 17 elements on the. The rare earth metals naturally have. Why Is It Called Rare Earth Metals.
From www.globalxetfs.com.au
Rare Earth Elements, Explained Global X ETFs Australia Why Is It Called Rare Earth Metals Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. But what exactly are ‘rare earth’ elements and just how rare are they? Rare earth elements are a collection of 17 elements on the. The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds. Why Is It Called Rare Earth Metals.
From www.studyiq.com
Rare Earth Elements, Metals, Minerals, Applications, Significance Why Is It Called Rare Earth Metals Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. But what exactly are ‘rare earth’ elements and just how rare are they? Rare earth elements are a collection of 17 elements on the. It’s significant that there are 15 rare earth elements: The rare earth elements are a. Why Is It Called Rare Earth Metals.
From wp.me
All About Rare Earth Metals [INFOGRAPHIC] Infographic List Why Is It Called Rare Earth Metals But what exactly are ‘rare earth’ elements and just how rare are they? The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. Rare earth elements are a collection of. Why Is It Called Rare Earth Metals.
From www.slideserve.com
PPT What Are Rare Earth Metals? PowerPoint Presentation, free download ID7233103 Why Is It Called Rare Earth Metals The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. Rare earth elements are a collection of 17 elements on the. But. Why Is It Called Rare Earth Metals.
From www.priyamstudycentre.com
Rare Earth Elements Metals, Definition, Properties, Uses Why Is It Called Rare Earth Metals The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. Rare earth elements are a collection of 17 elements on the. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. It’s significant that there are 15 rare earth elements:. Why Is It Called Rare Earth Metals.
From www.alamy.com
Rareearth elements, also known as rareearth metals, on the periodic table, with atomic numbers Why Is It Called Rare Earth Metals The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. But what exactly are ‘rare earth’ elements and just how rare are. Why Is It Called Rare Earth Metals.
From www.alamy.com
Rareearth elements, also known as rareearth metals in alphabetical order Stock Vector Image Why Is It Called Rare Earth Metals But what exactly are ‘rare earth’ elements and just how rare are they? Rare earth elements are a collection of 17 elements on the. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. It’s significant that there are 15 rare earth elements: The rare earth metals naturally have. Why Is It Called Rare Earth Metals.
From mpcomagnetics.com
12 Things You Didn’t Know About Rare Earth Metals MPCO Why Is It Called Rare Earth Metals But what exactly are ‘rare earth’ elements and just how rare are they? The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. It’s. Why Is It Called Rare Earth Metals.
From newseu.cgtn.com
What are rare earth metals and what are they used for? CGTN Why Is It Called Rare Earth Metals But what exactly are ‘rare earth’ elements and just how rare are they? The rare earth metals naturally have three positive charges and form incredibly strong ionic bonds with phosphate counterions, each. Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. The rare earth elements are a group. Why Is It Called Rare Earth Metals.
From www.hsmagnets.com
Rare Earth Elements & Metals By HSMAG Why Is It Called Rare Earth Metals Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. Rare earth elements are a collection of 17 elements on the. But. Why Is It Called Rare Earth Metals.
From www.youtube.com
Rare metals in the world Price of rare metals rare earth elements YouTube Why Is It Called Rare Earth Metals Chemistry students may recall that when electrons are added to an atom, they collect in groups or layers, called orbitals, which. The rare earth elements are a group of transition metals, found mainly in the first row below the periodic table (the lanthanide series), plus scandium and yttrium. It’s significant that there are 15 rare earth elements: The rare earth. Why Is It Called Rare Earth Metals.