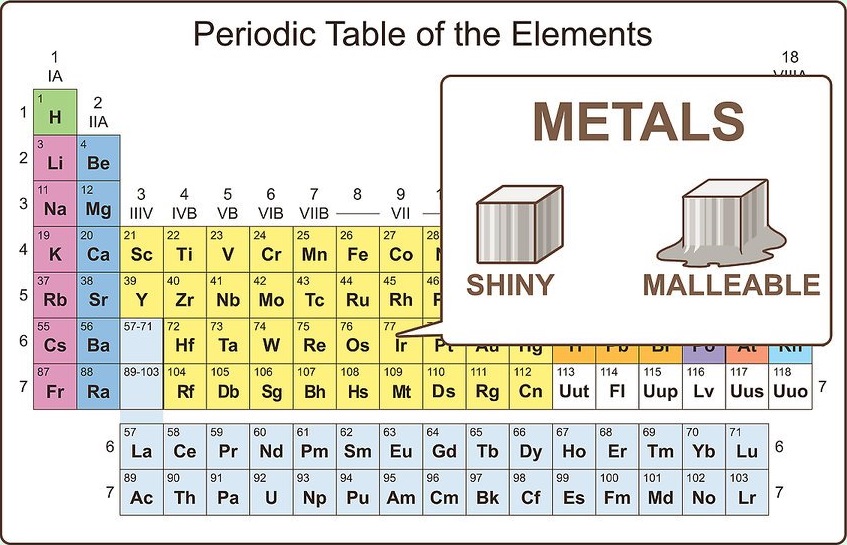
Brittle Elements . Metals tend to have similar. melting point of metals: This includes its properties, uses in everyday life, discovery, and more! Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. These are electronegative elements with high. in this article, you will learn all about the element beryllium. However, most boiling points are still quite high. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Of electricity and heat, and they are always lustrous (shiny. Since in ceramics the rows cannot slide, the.
from bucarotechelp.com
in this article, you will learn all about the element beryllium. Since in ceramics the rows cannot slide, the. melting point of metals: These are electronegative elements with high. Metals tend to have similar. Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Of electricity and heat, and they are always lustrous (shiny. This includes its properties, uses in everyday life, discovery, and more! However, most boiling points are still quite high.
Brief Description of the Chemical and Physical Properties of Elements
Brittle Elements melting point of metals: This includes its properties, uses in everyday life, discovery, and more! These are electronegative elements with high. Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. However, most boiling points are still quite high. Metals tend to have similar. in this article, you will learn all about the element beryllium. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. melting point of metals: Since in ceramics the rows cannot slide, the. Of electricity and heat, and they are always lustrous (shiny.
From www.flickr.com
Brittle All elements shot with an iPhone5. Edited on iPad … Flickr Brittle Elements in this article, you will learn all about the element beryllium. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. melting point of metals: However, most boiling points are still quite high. These are electronegative elements with high. Of electricity and heat, and they are. Brittle Elements.
From exowpciti.blob.core.windows.net
Brittleness Definition Of Terms at Harrison Johnson blog Brittle Elements This includes its properties, uses in everyday life, discovery, and more! in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. However, most boiling points are still quite high. melting point of metals: in this article, you will learn all about the element beryllium. These are. Brittle Elements.
From www.breakingatom.com
Brittle Definition Brittle Elements These are electronegative elements with high. melting point of metals: Metals tend to have similar. Of electricity and heat, and they are always lustrous (shiny. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. in this article, you will learn all about the element beryllium.. Brittle Elements.
From pediaa.com
Difference Between Ductile and Brittle Definition, Examples, Effect Brittle Elements in this article, you will learn all about the element beryllium. This includes its properties, uses in everyday life, discovery, and more! Metals tend to have similar. Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. These. Brittle Elements.
From www.researchgate.net
Brittle elastic damage constitutive relationship of elements under Brittle Elements Of electricity and heat, and they are always lustrous (shiny. melting point of metals: in this article, you will learn all about the element beryllium. This includes its properties, uses in everyday life, discovery, and more! in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing.. Brittle Elements.
From dxohukfiz.blob.core.windows.net
Brittle Definition Physics at Beulah Taylor blog Brittle Elements These are electronegative elements with high. Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. This includes its properties, uses in everyday life, discovery, and more! in this article, you will learn all about the element beryllium.. Brittle Elements.
From www.youtube.com
The Unique Properties of Solid Matter Brittleness & Malleability Brittle Elements in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Since in ceramics the rows cannot slide, the. Metals tend to have similar. in this article, you will learn all about the element beryllium. Of electricity and heat, and they are always lustrous (shiny. This includes its. Brittle Elements.
From www.researchgate.net
The elasticbrittle constitutive laws of element. Download Scientific Brittle Elements in this article, you will learn all about the element beryllium. melting point of metals: Since in ceramics the rows cannot slide, the. Of electricity and heat, and they are always lustrous (shiny. Metals tend to have similar. These are electronegative elements with high. However, most boiling points are still quite high. This includes its properties, uses in. Brittle Elements.
From sciencenotes.org
Free Printable Periodic Tables (PDF and PNG) Science Notes and Projects Brittle Elements These are electronegative elements with high. This includes its properties, uses in everyday life, discovery, and more! in this article, you will learn all about the element beryllium. However, most boiling points are still quite high. Since in ceramics the rows cannot slide, the. in metals, the sliding of rows of atoms results in slip, which allows the. Brittle Elements.
From www.slideshare.net
Periodic table of elements Brittle Elements These are electronegative elements with high. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. However, most boiling points are still quite high. Of electricity and heat, and they are always lustrous (shiny. Since in ceramics the rows cannot slide, the. Metals often have high melting and. Brittle Elements.
From howforkids.com
Examples of Brittle Materials HowForKids Brittle Elements Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. Metals tend to have similar. These are electronegative elements with high. Of electricity and heat, and they are always lustrous (shiny. in this article, you will learn all. Brittle Elements.
From pressbooks.bccampus.ca
Appendix C Periodic Table of the Elements from Open Stax Chemistry 1st Brittle Elements Since in ceramics the rows cannot slide, the. These are electronegative elements with high. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. This includes its properties, uses in everyday life, discovery, and more! Metals often have high melting and boiling points, but there are many exceptions. Brittle Elements.
From www.researchgate.net
Hierarchical models of series and parallel couplings of brittle Brittle Elements However, most boiling points are still quite high. Metals tend to have similar. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. melting point of metals: These are electronegative elements with high. Of electricity and heat, and they are always lustrous (shiny. Since in ceramics the. Brittle Elements.
From brainly.com
15 which element can be brittle or soft in a solid phase and is poor Brittle Elements These are electronegative elements with high. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. However, most boiling points are still quite high. Metals tend to have similar. Of electricity and heat, and they are always lustrous (shiny. Since in ceramics the rows cannot slide, the. . Brittle Elements.
From www.researchgate.net
6 The brittle star Amphiura filiformis. A) Aboral view of A Brittle Elements in this article, you will learn all about the element beryllium. Of electricity and heat, and they are always lustrous (shiny. These are electronegative elements with high. Since in ceramics the rows cannot slide, the. However, most boiling points are still quite high. melting point of metals: in metals, the sliding of rows of atoms results in. Brittle Elements.
From bucarotechelp.com
Brief Description of the Chemical and Physical Properties of Elements Brittle Elements Of electricity and heat, and they are always lustrous (shiny. in this article, you will learn all about the element beryllium. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Since in ceramics the rows cannot slide, the. This includes its properties, uses in everyday life,. Brittle Elements.
From blog.thepipingmart.com
Explain In Detail The Concept Of Brittleness ThePipingMart Blog Brittle Elements This includes its properties, uses in everyday life, discovery, and more! in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Since in ceramics the rows cannot slide, the. However, most boiling points are still quite high. melting point of metals: in this article, you will. Brittle Elements.
From geologylearn.blogspot.kr
Brittle Structures Learning Geology Brittle Elements This includes its properties, uses in everyday life, discovery, and more! in this article, you will learn all about the element beryllium. Metals tend to have similar. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Metals often have high melting and boiling points, but there. Brittle Elements.
From www.chemistry-teaching-resources.com
chemistry picture Brittle Elements Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. Since in ceramics the rows cannot slide, the. However, most boiling points are still quite high. These are electronegative elements with high. in this article, you will learn. Brittle Elements.
From www.gauthmath.com
Solved Elements that are dull, brittle, often are gases at room Brittle Elements However, most boiling points are still quite high. Of electricity and heat, and they are always lustrous (shiny. Since in ceramics the rows cannot slide, the. Metals tend to have similar. melting point of metals: These are electronegative elements with high. This includes its properties, uses in everyday life, discovery, and more! in metals, the sliding of rows. Brittle Elements.
From www.researchgate.net
Brittle elastic damage constitutive relationship of elements under Brittle Elements This includes its properties, uses in everyday life, discovery, and more! in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. in this article, you will learn all about the element beryllium. Of electricity and heat, and they are always lustrous (shiny. Since in ceramics the rows. Brittle Elements.
From slideplayer.com
Periodic Table of Elements ppt download Brittle Elements Of electricity and heat, and they are always lustrous (shiny. However, most boiling points are still quite high. Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. Metals tend to have similar. Since in ceramics the rows cannot. Brittle Elements.
From hotelsryte.weebly.com
hotelsryte Blog Brittle Elements melting point of metals: in this article, you will learn all about the element beryllium. Since in ceramics the rows cannot slide, the. Metals tend to have similar. Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting. Brittle Elements.
From www.slideserve.com
PPT How to Read the Periodic Table PowerPoint Presentation, free Brittle Elements Metals tend to have similar. melting point of metals: in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. However, most boiling points are still quite high. These are electronegative elements with high. Metals often have high melting and boiling points, but there are many exceptions to. Brittle Elements.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2975896 Brittle Elements However, most boiling points are still quite high. These are electronegative elements with high. Metals tend to have similar. Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. in this article, you will learn all about the. Brittle Elements.
From www.youtube.com
Ductile and Brittle Materials by stress strain curve YouTube Brittle Elements Since in ceramics the rows cannot slide, the. These are electronegative elements with high. Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. This includes its properties, uses in everyday life, discovery, and more! However, most boiling points. Brittle Elements.
From slideplayer.com
History and Arrangement of the Periodic Table ppt download Brittle Elements Of electricity and heat, and they are always lustrous (shiny. in this article, you will learn all about the element beryllium. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. This includes its properties, uses in everyday life, discovery, and more! However, most boiling points are. Brittle Elements.
From www.wikiwand.com
Brittleductile transition zone Wikiwand Brittle Elements Of electricity and heat, and they are always lustrous (shiny. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. These are electronegative elements with high. melting point of metals: Metals often have high melting and boiling points, but there are many exceptions to the melting point,. Brittle Elements.
From www.pinterest.com
Learn BRITTLE MATERIARS in 3 minutes Brittle, Material, Art gallery Brittle Elements This includes its properties, uses in everyday life, discovery, and more! in this article, you will learn all about the element beryllium. Of electricity and heat, and they are always lustrous (shiny. Since in ceramics the rows cannot slide, the. Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium,. Brittle Elements.
From www.youtube.com
Lesson 4 DUCTILE TO BRITTLE TRANSITION CONDITIONS YouTube Brittle Elements Metals tend to have similar. Since in ceramics the rows cannot slide, the. These are electronegative elements with high. Of electricity and heat, and they are always lustrous (shiny. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Metals often have high melting and boiling points, but. Brittle Elements.
From howforkids.com
15 Examples of Brittle Materials HowForKids Brittle Elements Metals tend to have similar. in this article, you will learn all about the element beryllium. in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. This includes its properties, uses in everyday life, discovery, and more! However, most boiling points are still quite high. melting. Brittle Elements.
From www.thoughtco.com
First 20 Elements of the Periodic Table Brittle Elements Metals often have high melting and boiling points, but there are many exceptions to the melting point, like cesium, gallium, mercury, rubidium and tin which all have fairly low melting points. in this article, you will learn all about the element beryllium. This includes its properties, uses in everyday life, discovery, and more! Metals tend to have similar. . Brittle Elements.
From fr.aliexpress.com
Métal élément Bi99.99 Brittle Bismuth lingots, Multifonctionnel Bismuth Brittle Elements melting point of metals: Of electricity and heat, and they are always lustrous (shiny. These are electronegative elements with high. in this article, you will learn all about the element beryllium. Metals tend to have similar. Since in ceramics the rows cannot slide, the. in metals, the sliding of rows of atoms results in slip, which allows. Brittle Elements.
From www.luciteria.com
Beryllium metal 99.5 — Luciteria Brittle Elements This includes its properties, uses in everyday life, discovery, and more! Metals tend to have similar. However, most boiling points are still quite high. melting point of metals: in this article, you will learn all about the element beryllium. These are electronegative elements with high. Metals often have high melting and boiling points, but there are many exceptions. Brittle Elements.
From blog.thepipingmart.com
Is Zinc a Brittle Metal? Brittle Elements in metals, the sliding of rows of atoms results in slip, which allows the metal to deform plastically instead of fracturing. Of electricity and heat, and they are always lustrous (shiny. in this article, you will learn all about the element beryllium. Metals tend to have similar. These are electronegative elements with high. However, most boiling points are. Brittle Elements.