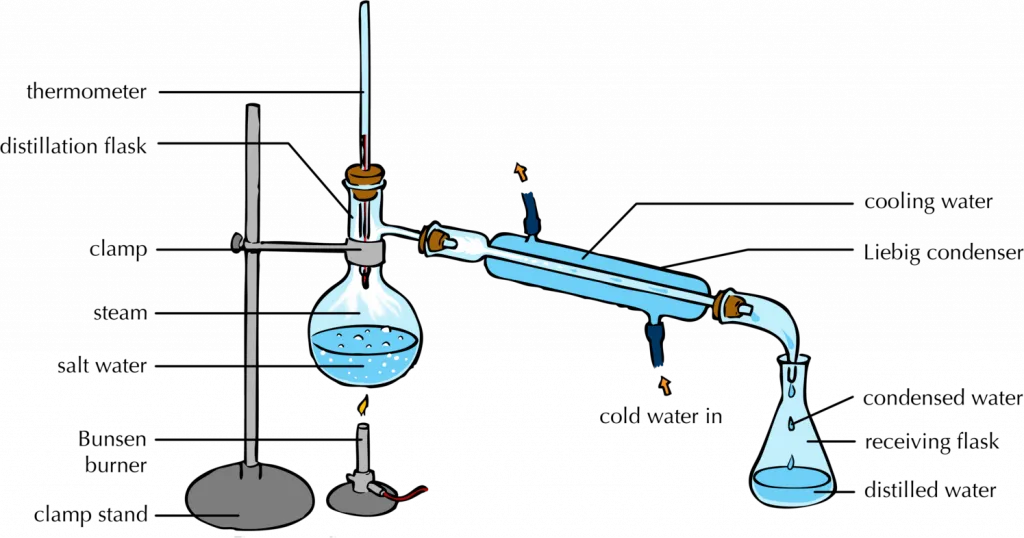
Filtration And Distillation Difference . Distillation is a purification process where one. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Distillation (as discussed in analysis:. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. distillation is an effective method to separate mixtures comprised of two or more pure liquids. Evaporation removes a liquid from a solution to leave a solid material. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Filtration separates solids of different sizes. Lead students through an investigation of the different allotropes of. distillation takes advantage of differences in boiling points.
from www.theengineersperspectives.com
Distillation is a purification process where one. Distillation (as discussed in analysis:. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Evaporation removes a liquid from a solution to leave a solid material. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. distillation is an effective method to separate mixtures comprised of two or more pure liquids. distillation takes advantage of differences in boiling points. Filtration separates solids of different sizes. Lead students through an investigation of the different allotropes of. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,.
How Does Simple Distillation Work? The Engineer's Perspective
Filtration And Distillation Difference the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Filtration separates solids of different sizes. Lead students through an investigation of the different allotropes of. Distillation is a purification process where one. distillation takes advantage of differences in boiling points. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Distillation (as discussed in analysis:. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation is an effective method to separate mixtures comprised of two or more pure liquids. Evaporation removes a liquid from a solution to leave a solid material. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] Filtration And Distillation Difference there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Distillation is a purification process where one. Filtration separates solids of different sizes. Distillation (as discussed in analysis:. distillation takes advantage of differences in boiling points. Lead students through an investigation of the different allotropes of. distillation is the use of heat to separate. Filtration And Distillation Difference.
From exooxhrub.blob.core.windows.net
Distillation Diagram With Labels at Hipolito Jackson blog Filtration And Distillation Difference distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation is a purification process where one. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. there are different. Filtration And Distillation Difference.
From exoibxrus.blob.core.windows.net
Are Filtration And Distillation The Same at Tom Estey blog Filtration And Distillation Difference distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation is a purification process where one. Lead students through an investigation of the different allotropes of. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either. Filtration And Distillation Difference.
From www.theperfectwater.com
Know the Differences Between Filtered, Distilled and Tap Water Filtration And Distillation Difference there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Distillation is a purification process where one. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Lead students through an investigation of the different allotropes of. Filtration separates solids of different sizes. Distillation. Filtration And Distillation Difference.
From www.yaclass.in
Simple distillation — lesson. Science CBSE, Class 9. Filtration And Distillation Difference Filtration separates solids of different sizes. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. Distillation is a purification process where one. Distillation (as discussed in analysis:. Lead students through an investigation of the different allotropes. Filtration And Distillation Difference.
From dxondimvq.blob.core.windows.net
Filtration And Distillation Method at Mary Bowser blog Filtration And Distillation Difference Filtration separates solids of different sizes. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Distillation (as discussed in analysis:. distillation is the use of heat to separate the components of a. Filtration And Distillation Difference.
From pediaa.com
Difference Between Distilled Water and Purified Water Definition Filtration And Distillation Difference Lead students through an investigation of the different allotropes of. Filtration separates solids of different sizes. distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation (as discussed in analysis:. Evaporation removes a liquid from a solution to leave a solid material. distillation is the use of heat to separate the components. Filtration And Distillation Difference.
From hadoma.com
Sterile Water Vs Distilled Water Know The Difference (2022) Filtration And Distillation Difference Evaporation removes a liquid from a solution to leave a solid material. distillation is an effective method to separate mixtures comprised of two or more pure liquids. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation takes advantage of differences in boiling points. Distillation is a purification process where one. Lead students. Filtration And Distillation Difference.
From pediaa.com
Difference Between Fractional Distillation and Simple Distillation Filtration And Distillation Difference there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Filtration separates solids of different sizes. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. Lead students through an investigation of the. Filtration And Distillation Difference.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Filtration And Distillation Difference there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation is an effective method to separate mixtures comprised of two or more pure liquids. Filtration separates solids of different sizes. Lead students through an investigation of the different allotropes of. Distillation (as discussed in analysis:. distillation takes advantage of differences in boiling points.. Filtration And Distillation Difference.
From www.premierh2o.com
Difference Between Distilled, Tap, and Filtered Water Filtration And Distillation Difference Filtration separates solids of different sizes. Evaporation removes a liquid from a solution to leave a solid material. distillation takes advantage of differences in boiling points. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Distillation is a purification process where one. distillation is an. Filtration And Distillation Difference.
From www.chemicals.co.uk
What’s the Difference Between Distilled & Ultrapure Water? The Filtration And Distillation Difference Evaporation removes a liquid from a solution to leave a solid material. Distillation is a purification process where one. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Lead students through an investigation of the different allotropes of. distillation is an effective method to separate mixtures. Filtration And Distillation Difference.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Filtration And Distillation Difference distillation is an effective method to separate mixtures comprised of two or more pure liquids. Filtration separates solids of different sizes. distillation takes advantage of differences in boiling points. Distillation is a purification process where one. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of. Filtration And Distillation Difference.
From byjus.com
What is the difference between distillation, distillation under reduced Filtration And Distillation Difference the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Evaporation removes a liquid from a solution to leave a solid material. Filtration separates solids of different sizes. Distillation is a purification process where one. distillation is an effective method to separate mixtures comprised of two or. Filtration And Distillation Difference.
From waterseer.org
Distilled Water Vs Deionized Water Know The Real Difference Filtration And Distillation Difference there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation takes advantage of differences in boiling points. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Filtration separates solids of different sizes. distillation is the use of heat to separate. Filtration And Distillation Difference.
From chemicalengineeringworld.com
Types of Distillation Chemical Engineering World Filtration And Distillation Difference the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Distillation is a purification process where one. Filtration separates solids of different sizes. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a. Filtration And Distillation Difference.
From microbiologyjournal.org
Analytical Review on Membrane Water Filter using Different Materials to Filtration And Distillation Difference there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Filtration separates solids of different sizes. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Evaporation removes a liquid from a solution to leave a solid material. Distillation (as discussed in analysis:. . Filtration And Distillation Difference.
From www.geeksforgeeks.org
Methods of Separation Various Separation Techniques Filtration And Distillation Difference Distillation is a purification process where one. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Evaporation removes a liquid from a solution to leave a solid material. Filtration separates solids of different sizes. distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation (as discussed in analysis:.. Filtration And Distillation Difference.
From chem.libretexts.org
3.6 Changes in Matter Physical and Chemical Changes Chemistry Filtration And Distillation Difference Lead students through an investigation of the different allotropes of. Evaporation removes a liquid from a solution to leave a solid material. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation (as discussed in analysis:. distillation is. Filtration And Distillation Difference.
From thecontentauthority.com
Distillation vs Filtration Decoding Common Word MixUps Filtration And Distillation Difference distillation takes advantage of differences in boiling points. Filtration separates solids of different sizes. Evaporation removes a liquid from a solution to leave a solid material. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Distillation (as discussed in analysis:. Distillation is a purification process where one. Lead students through an investigation of the. Filtration And Distillation Difference.
From www.chemicalslearning.com
Difference Between Distillation and Extraction Process Filtration And Distillation Difference Lead students through an investigation of the different allotropes of. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Filtration separates solids of different sizes. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of. Filtration And Distillation Difference.
From waterfiltersadvisor.com
Distilled Vs. Purified Water (We Explain The Differences Filtration And Distillation Difference Lead students through an investigation of the different allotropes of. distillation takes advantage of differences in boiling points. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either. Filtration And Distillation Difference.
From exoibxrus.blob.core.windows.net
Are Filtration And Distillation The Same at Tom Estey blog Filtration And Distillation Difference there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. Lead students through an investigation of the different allotropes of. distillation takes. Filtration And Distillation Difference.
From dxondimvq.blob.core.windows.net
Filtration And Distillation Method at Mary Bowser blog Filtration And Distillation Difference Evaporation removes a liquid from a solution to leave a solid material. Filtration separates solids of different sizes. distillation takes advantage of differences in boiling points. Distillation (as discussed in analysis:. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a. Filtration And Distillation Difference.
From www.askdifference.com
Distillation vs. Filtration — What’s the Difference? Filtration And Distillation Difference distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. Evaporation removes a liquid from a solution to leave a solid material. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. . Filtration And Distillation Difference.
From quenchwater.com
Types of Water Filters and How They Work I Quench Water Filtration And Distillation Difference there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Lead students through an investigation of the different allotropes of. distillation is an effective method to separate mixtures comprised of two or more pure liquids. Filtration separates solids of different sizes. the choice between distillation and filtration depends on the nature of the mixture. Filtration And Distillation Difference.
From www.slideserve.com
PPT CHEMISTRY I PowerPoint Presentation, free download ID3104697 Filtration And Distillation Difference Distillation (as discussed in analysis:. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Evaporation removes a liquid from a solution to leave a solid material. distillation takes advantage of differences in boiling points. distillation is the use of heat to separate the components of. Filtration And Distillation Difference.
From microbenotes.com
Water Distiller Principle, Parts, Types, Uses, Examples Filtration And Distillation Difference distillation takes advantage of differences in boiling points. distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. the choice between distillation and filtration depends on the nature of the mixture and the desired purity. Filtration And Distillation Difference.
From online-learning-college.com
Separating mixtures Distillation, Filtration & Crystallisation Filtration And Distillation Difference Lead students through an investigation of the different allotropes of. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Filtration separates solids of different sizes. distillation takes advantage of differences in boiling points. distillation is an effective method to separate mixtures comprised of two or. Filtration And Distillation Difference.
From pediaa.com
Difference Between Distillation and Extraction Definition, Technique Filtration And Distillation Difference distillation takes advantage of differences in boiling points. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Lead students through an investigation of the different allotropes of. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Distillation (as discussed in analysis:.. Filtration And Distillation Difference.
From www.aquaportail.com
Distillation définition et explications AquaPortail Filtration And Distillation Difference the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Filtration separates solids of different sizes. Distillation is a purification process where one. distillation takes advantage of differences in boiling points. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation. Filtration And Distillation Difference.
From chemistryismyjam.com
Science and Measurement Chemistry Is My Jam! Filtration And Distillation Difference Filtration separates solids of different sizes. distillation takes advantage of differences in boiling points. Distillation (as discussed in analysis:. Evaporation removes a liquid from a solution to leave a solid material. Distillation is a purification process where one. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. distillation is the use of heat. Filtration And Distillation Difference.
From pediaa.com
Difference Between Distillation and Extraction Definition, Technique Filtration And Distillation Difference distillation takes advantage of differences in boiling points. distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation is a purification process where one. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation,. Distillation (as discussed in analysis:. Evaporation removes a liquid from a solution to leave. Filtration And Distillation Difference.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] Filtration And Distillation Difference distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by. Filtration separates solids of different sizes. Distillation (as discussed in analysis:. distillation is an effective method to separate mixtures comprised of two or more pure liquids.. Filtration And Distillation Difference.
From www.watersmartsystems.com
Distilled Water vs Water Softener vs Filtered Water Detailed Filtration And Distillation Difference distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation (as discussed in analysis:. the choice between distillation and filtration depends on the nature of the mixture and the desired purity of the final product. Evaporation removes a liquid from a solution to leave a solid material. distillation takes advantage of. Filtration And Distillation Difference.