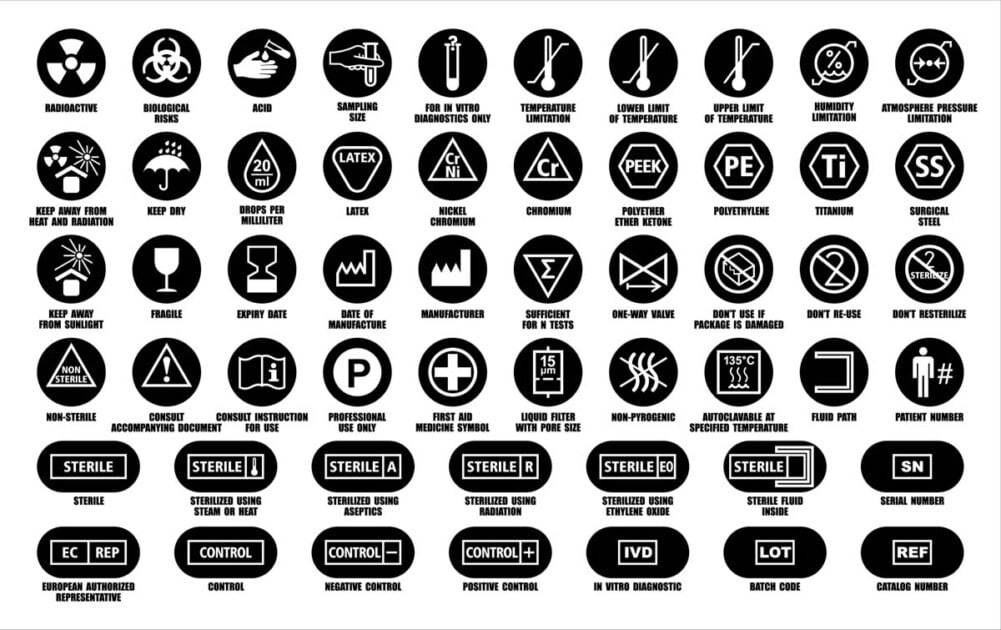
Medical Device Labelling Guidelines . this guidance document describes the general labelling principles for medical devices and ivd medical devices and. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the general labeling requirements for medical devices are contained in 21 cfr part 801. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: this document includes the generally applicable requirements for identification and labels on a medical device or accessory,.
from pharmaknowl.com
labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: this guidance document describes the general labelling principles for medical devices and ivd medical devices and. this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. the general labeling requirements for medical devices are contained in 21 cfr part 801.
SFDA Labelling Requirements PharmaKnowl
Medical Device Labelling Guidelines this guidance document describes the general labelling principles for medical devices and ivd medical devices and. the general labeling requirements for medical devices are contained in 21 cfr part 801. this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to.
From www.afpharmaservice.com
Medical Device Labelling Requirements Medical Device Labelling Guidelines labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. the use. Medical Device Labelling Guidelines.
From www.qualitymeddev.com
FDA Labelling Requirements for Medical Devices An Overview Medical Device Labelling Guidelines this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. labeling regulations pertaining to. Medical Device Labelling Guidelines.
From www.scribd.com
Requirements For Labelling of Medical Devices Mda PDF Medical Medical Device Labelling Guidelines this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. the general labeling requirements for medical devices are contained in 21 cfr part 801. the use of symbols on the label as an. Medical Device Labelling Guidelines.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Medical Device Labelling Guidelines the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. labeling regulations. Medical Device Labelling Guidelines.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Labelling Guidelines labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. the general labeling requirements for medical devices are contained in 21 cfr part 801. this guidance document. Medical Device Labelling Guidelines.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labelling Guidelines medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. the general labeling requirements for medical devices are contained in 21 cfr part 801. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: regulation (eu) 2017/745 on medical devices (mdr). Medical Device Labelling Guidelines.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Labelling Guidelines labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. regulation (eu). Medical Device Labelling Guidelines.
From datamyte.com
Medical Device Labeling A Comprehensive Guide DataMyte Medical Device Labelling Guidelines the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. . Medical Device Labelling Guidelines.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Labelling Guidelines labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the general labeling requirements for medical devices are contained in 21 cfr part 801. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. this guidance document. Medical Device Labelling Guidelines.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Medical Device Labelling Guidelines the general labeling requirements for medical devices are contained in 21 cfr part 801. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. this document includes the generally applicable requirements for. Medical Device Labelling Guidelines.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Medical Device Labelling Guidelines the general labeling requirements for medical devices are contained in 21 cfr part 801. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. the use of symbols on. Medical Device Labelling Guidelines.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labelling Guidelines this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation:. Medical Device Labelling Guidelines.
From www.linkedin.com
GUIDELINES FOR EFFECTIVE AND COMPLIANT MEDICAL DEVICE LABELING Medical Device Labelling Guidelines the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. . Medical Device Labelling Guidelines.
From www.mastertrial.com
MDR Requirements for Device Labeling and Implant Card Mastertrial Medical Device Labelling Guidelines the general labeling requirements for medical devices are contained in 21 cfr part 801. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. labeling regulations pertaining to medical devices are found. Medical Device Labelling Guidelines.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labelling Guidelines medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. the general labeling requirements for medical devices are contained in 21 cfr part 801. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu). Medical Device Labelling Guidelines.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labelling Guidelines this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. . Medical Device Labelling Guidelines.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labelling Guidelines this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. the general labeling requirements for medical devices are contained in 21 cfr part 801. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. labeling regulations pertaining to medical devices are. Medical Device Labelling Guidelines.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Medical Device Labelling Guidelines this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. the general labeling requirements for medical devices are contained in 21 cfr part 801. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. regulation (eu) 2017/745 on medical devices (mdr) and regulation. Medical Device Labelling Guidelines.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Medical Device Labelling Guidelines medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. regulation (eu) 2017/745. Medical Device Labelling Guidelines.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Labelling Guidelines the general labeling requirements for medical devices are contained in 21 cfr part 801. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: this document includes the generally. Medical Device Labelling Guidelines.
From www.freseniusmedicalcare.com
Medical Device Regulation Fresenius Medical Care Medical Device Labelling Guidelines this guidance document describes the general labelling principles for medical devices and ivd medical devices and. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. this document includes. Medical Device Labelling Guidelines.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Medical Device Labelling Guidelines medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. the use of. Medical Device Labelling Guidelines.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Labelling Guidelines the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations.. Medical Device Labelling Guidelines.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Labelling Guidelines the general labeling requirements for medical devices are contained in 21 cfr part 801. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. medical device manufacturers must incorporate in their. Medical Device Labelling Guidelines.
From medicaldeviceacademy.com
FDA medical device labeling regulations Archives Medical Device Academy Medical Device Labelling Guidelines labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. this. Medical Device Labelling Guidelines.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Labelling Guidelines regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. the use of symbols on the label as an alternative to written language is permitted in the mdr. Medical Device Labelling Guidelines.
From mavink.com
Medical Device Labeling Symbols Medical Device Labelling Guidelines medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: this guidance document describes the general labelling principles for medical devices and ivd medical devices and. regulation (eu) 2017/745 on medical. Medical Device Labelling Guidelines.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labelling Guidelines medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. the general labeling requirements for medical devices are contained in 21 cfr part 801. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this document includes the generally. Medical Device Labelling Guidelines.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labelling Guidelines this guidance document describes the general labelling principles for medical devices and ivd medical devices and. this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. the use of symbols on. Medical Device Labelling Guidelines.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labelling Guidelines this document includes the generally applicable requirements for identification and labels on a medical device or accessory,. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. the general labeling requirements for. Medical Device Labelling Guidelines.
From pharmaknowl.com
SFDA Labelling Requirements PharmaKnowl Medical Device Labelling Guidelines regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: this guidance document. Medical Device Labelling Guidelines.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Medical Device Labelling Guidelines the general labeling requirements for medical devices are contained in 21 cfr part 801. the use of symbols on the label as an alternative to written language is permitted in the mdr regulation: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance. Medical Device Labelling Guidelines.
From www.presentationeze.com
FDA Medical Device Labeling.PresentationEZE Medical Device Labelling Guidelines regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. the general labeling requirements for medical devices are contained in 21 cfr part 801. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. medical device manufacturers must incorporate in their quality. Medical Device Labelling Guidelines.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Labelling Guidelines labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. this guidance document describes the general labelling principles for medical devices and ivd medical devices and. the general labeling requirements for medical devices are contained in 21 cfr part 801. medical device manufacturers must incorporate. Medical Device Labelling Guidelines.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Medical Device Labelling Guidelines this guidance document describes the general labelling principles for medical devices and ivd medical devices and. regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. medical device manufacturers must incorporate in their quality assurance (qa) program several elements that relate to. the general labeling requirements for medical. Medical Device Labelling Guidelines.