Dilution Method Of Drugs . Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. Few use a commercially available prefilled 'flush' syringe to dilute medications. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. The following is a brief explanation of some ways of calculating. Keeping the mass of the solute (drug) constant. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. There are many ways of expressing concentration and dilution.
from labpedia.net
To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. The following is a brief explanation of some ways of calculating. Keeping the mass of the solute (drug) constant. Few use a commercially available prefilled 'flush' syringe to dilute medications. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. There are many ways of expressing concentration and dilution.
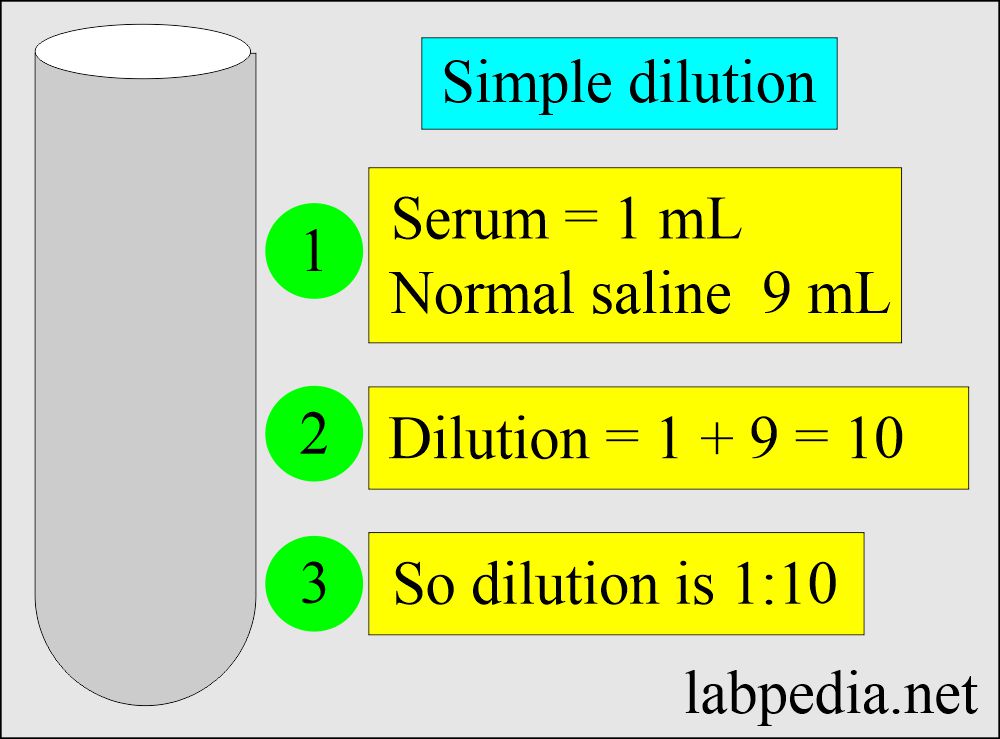
Solutions Part 1 Solutions Preparation used in Clinical Laboratory
Dilution Method Of Drugs The following is a brief explanation of some ways of calculating. The following is a brief explanation of some ways of calculating. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Few use a commercially available prefilled 'flush' syringe to dilute medications. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Keeping the mass of the solute (drug) constant. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. There are many ways of expressing concentration and dilution.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. The following is a brief explanation of some ways of calculating. Few use a commercially available prefilled 'flush' syringe to dilute medications. Dilution of a drug solution can be accomplished by changing the volume of the final. Dilution Method Of Drugs.
From ar.inspiredpencil.com
Serial Dilution Diagram Dilution Method Of Drugs There are many ways of expressing concentration and dilution. The following is a brief explanation of some ways of calculating. Few use a commercially available prefilled 'flush' syringe to dilute medications. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can. Dilution Method Of Drugs.
From ar.inspiredpencil.com
Serial Dilution Diagram Dilution Method Of Drugs The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. Few use a. Dilution Method Of Drugs.
From labrobot.com
Dilutions décimales Microbiologie alimentaire Système Dilucup Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. There are many ways of expressing concentration and dilution. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by. Dilution Method Of Drugs.
From www.researchgate.net
Agar dilution and broth macrodilution protocols for antimicrobial Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. Keeping the mass of the solute (drug) constant. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. The aliquot method consists of measuring out a small amount of drug,. Dilution Method Of Drugs.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA Dilution Method Of Drugs The following is a brief explanation of some ways of calculating. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Few use a commercially available prefilled 'flush' syringe to dilute medications. Dilution of a drug. Dilution Method Of Drugs.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis Dilution Method Of Drugs Keeping the mass of the solute (drug) constant. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Few use a commercially available prefilled 'flush' syringe to dilute medications. Dilution of a drug solution. Dilution Method Of Drugs.
From www.mdpi.com
Antibiotics Free FullText Antimicrobial Susceptibility Testing A Dilution Method Of Drugs To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. There. Dilution Method Of Drugs.
From www.researchgate.net
Scheme of the agar dilution method. (Created with Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. The following is a brief explanation of some ways of calculating. There are many. Dilution Method Of Drugs.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Keeping the mass of the solute (drug) constant. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. There are many ways of expressing concentration and dilution. Dilution of a. Dilution Method Of Drugs.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Method Of Drugs The following is a brief explanation of some ways of calculating. Few use a commercially available prefilled 'flush' syringe to dilute medications. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the nal. Dilution Method Of Drugs.
From slideplayer.com
Chapter 8 Solutions 8.5 Molarity and Dilution. ppt download Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. Keeping. Dilution Method Of Drugs.
From pharmacyscope.com
Methods of isolation of pure culture Pharmacy Scope Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Keeping the mass of the solute (drug) constant. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. There are many ways of expressing concentration and dilution. Dilution. Dilution Method Of Drugs.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Method Of Drugs To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. Dilution. Dilution Method Of Drugs.
From www.mdpi.com
Materials Free FullText Polymeric Micelles of Biodegradable Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Keeping the mass of the solute (drug) constant. The following is a brief explanation of some ways of calculating. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. Few. Dilution Method Of Drugs.
From www.researchgate.net
Procedures of serial dilution preparation Download Scientific Diagram Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. The following is a brief explanation of some ways of calculating. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. There are many. Dilution Method Of Drugs.
From www.researchgate.net
Agar dilution method with NPs. Download Scientific Diagram Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. There are many. Dilution Method Of Drugs.
From www.researchgate.net
(PDF) Assessment of relative Salty taste of Unani drugs by serial Dilution Method Of Drugs To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping. Dilution Method Of Drugs.
From emergencypedia.com
The Solution to Dilution EmergencyPedia Dilution Method Of Drugs Few use a commercially available prefilled 'flush' syringe to dilute medications. There are many ways of expressing concentration and dilution. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while.. Dilution Method Of Drugs.
From chemistnotes.com
Isolation of Bacteria Easy Procedure Chemistry Notes Dilution Method Of Drugs To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. The following is a brief explanation of some ways of calculating. Keeping the mass of the solute (drug). Dilution Method Of Drugs.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube Dilution Method Of Drugs Few use a commercially available prefilled 'flush' syringe to dilute medications. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be. Dilution Method Of Drugs.
From www.freepik.com
Premium Vector Serial Dilutions science vector illustration infographic Dilution Method Of Drugs To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. The following is a brief explanation of some ways of calculating. Keeping the mass of the solute (drug) constant. Few use a commercially available. Dilution Method Of Drugs.
From www.reddit.com
Serial Dilution in Microbiology Definition, Formula, Procedure and Dilution Method Of Drugs To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Few use a commercially available prefilled 'flush' syringe to dilute medications. Dilution of a drug solution can be accomplished by changing the volume of. Dilution Method Of Drugs.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Method Of Drugs To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. There are many ways of expressing concentration and dilution. Few use a commercially available prefilled 'flush' syringe to. Dilution Method Of Drugs.
From www.researchgate.net
Names, routes of administration, and pharmacology for the 10 studied Dilution Method Of Drugs The following is a brief explanation of some ways of calculating. Keeping the mass of the solute (drug) constant. Few use a commercially available prefilled 'flush' syringe to dilute medications. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. There are many ways of expressing concentration. Dilution Method Of Drugs.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilution Method Of Drugs To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. There are many ways of expressing concentration and dilution. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by. Dilution Method Of Drugs.
From www.researchgate.net
Illustration of the agar dilution method the drug is the antimicrobial Dilution Method Of Drugs The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Few use a commercially available prefilled 'flush' syringe to dilute medications. Keeping the mass of the solute (drug) constant. There are many ways of expressing concentration and dilution. The following is a brief explanation of some ways of calculating. Dilution of a drug. Dilution Method Of Drugs.
From www.researchgate.net
Illustration of the agar dilution method the drug is the antimicrobial Dilution Method Of Drugs Few use a commercially available prefilled 'flush' syringe to dilute medications. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. The following is a brief explanation of some ways of calculating. There are many ways of expressing concentration and dilution. The aliquot method consists of measuring. Dilution Method Of Drugs.
From www.nclexquiz.com
The nurse's quick guide to I.V. drug calculations NCLEX Quiz Dilution Method Of Drugs Keeping the mass of the solute (drug) constant. The following is a brief explanation of some ways of calculating. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be. Dilution Method Of Drugs.
From www.vrogue.co
What Is Serial Dilution Method And How To Calculate S vrogue.co Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Few use a commercially available prefilled 'flush' syringe to dilute medications. There are many ways of expressing concentration and dilution. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must. Dilution Method Of Drugs.
From www.slideshare.net
Antimicrobial susceptibility test and assay bls 206 Dilution Method Of Drugs Few use a commercially available prefilled 'flush' syringe to dilute medications. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by. Dilution Method Of Drugs.
From www.researchgate.net
Broth dilution method with NPs. Download Scientific Diagram Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. Dilution of a drug solution can be accomplished by changing the volume of the final solution while keeping the mass of the solute. There are many ways of expressing concentration and dilution. Dilution of a drug solution. Dilution Method Of Drugs.
From www.researchgate.net
Method of dilution of the solution to obtain a glucose calibration Dilution Method Of Drugs Dilution of a drug solution can be accomplished by changing the volume of the nal solution while. The following is a brief explanation of some ways of calculating. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. There are many ways of expressing concentration and dilution. Dilution of a drug. Dilution Method Of Drugs.
From www.researchgate.net
Dilution method for the isolation of soil fungi. Download Scientific Dilution Method Of Drugs Few use a commercially available prefilled 'flush' syringe to dilute medications. The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Keeping the mass of the solute (drug) constant. Dilution of a drug solution. Dilution Method Of Drugs.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Method Of Drugs The aliquot method consists of measuring out a small amount of drug, by diluting a larger amount. Keeping the mass of the solute (drug) constant. To understand how dilutions work, certain basic assumptions for the properties of fluids such as water must be made. Dilution of a drug solution can be accomplished by changing the volume of the nal solution. Dilution Method Of Drugs.