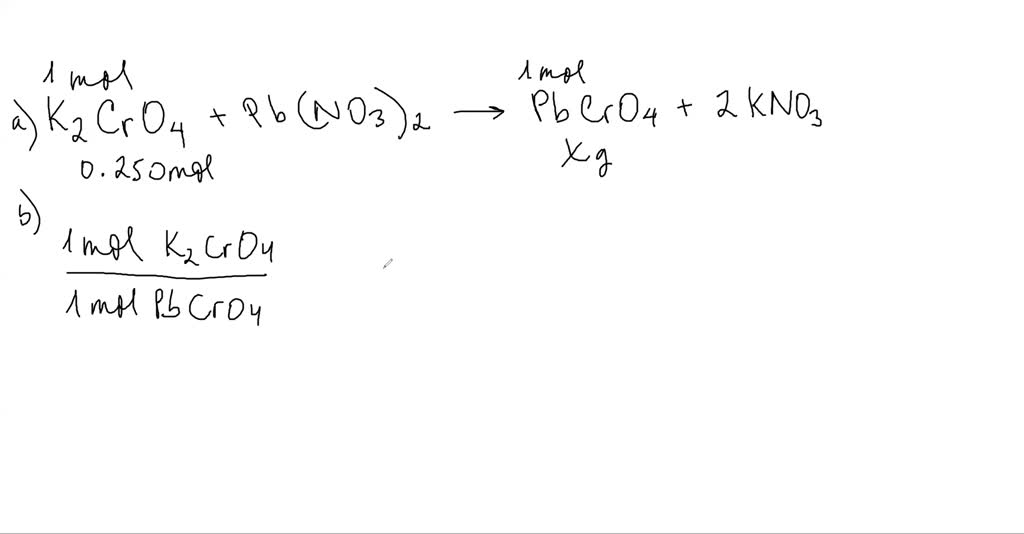Lead Ii Nitrate And Sodium Hydroxide Balanced Equation . aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. The balanced equation will be calculated along. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. enter an equation of an ionic chemical equation and press the balance button. use the calculator below to balance chemical equations and determine the type of reaction (instructions). How do you write the balanced equation. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate.
from www.numerade.com
The balanced equation will be calculated along. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. The products of the reaction are an aqueous solution of. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. How do you write the balanced equation. use the calculator below to balance chemical equations and determine the type of reaction (instructions). enter an equation of an ionic chemical equation and press the balance button. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion.
SOLVEDWhen a solution of lead(II) nitrate is mixed with a solution of sodium chromate, a yellow
Lead Ii Nitrate And Sodium Hydroxide Balanced Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. The products of the reaction are an aqueous solution of. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. How do you write the balanced equation. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. use the calculator below to balance chemical equations and determine the type of reaction (instructions).
From giothzckz.blob.core.windows.net
Lead 2 Hydroxide Formula at Frank Spring blog Lead Ii Nitrate And Sodium Hydroxide Balanced Equation How do you write the balanced equation. The products of the reaction are an aqueous solution of. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. lead (ii) nitrate reacts with potassium iodide to form lead (ii). Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved Consider the reaction of aqueous lead (II) nitrate Lead Ii Nitrate And Sodium Hydroxide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. aqueous solutions. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free download ID4566823 Lead Ii Nitrate And Sodium Hydroxide Balanced Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). The balanced equation will be calculated along. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved 5. Lead(II) nitrate + sodium hydroxide → Balanced Lead Ii Nitrate And Sodium Hydroxide Balanced Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). enter an equation of an ionic chemical equation and press the balance button. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide,. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.youtube.com
How to Balance Pb(NO3)2 + Na3PO4 = Pb3(PO4)2 + NaNO3 Lead (II) nitrate + Sodium phosphate Lead Ii Nitrate And Sodium Hydroxide Balanced Equation The balanced equation will be calculated along. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. How do you write the balanced equation. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Write the balanced net ionic equation for the reaction of aqueous sodium sulfate with Lead Ii Nitrate And Sodium Hydroxide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. enter an equation of an ionic chemical equation and press the balance button. How do you write the balanced equation. The balanced equation will be calculated along. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From giovauxoc.blob.core.windows.net
Lead(Ii) Nitrate And Sodium Chloride Empirical Formula at Lillian Dietrich blog Lead Ii Nitrate And Sodium Hydroxide Balanced Equation when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. use the calculator below to balance chemical equations and determine the type of reaction (instructions). Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From slideplayer.com
Chapter 9 Balancing Equations. Parts of a Reaction H 2 SO 3 (aq) H 2 O (l) + SO 2 (g Lead Ii Nitrate And Sodium Hydroxide Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. How do you write the balanced equation. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Kbr (s) → k. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.chegg.com
Write the balanced chemical equation for each of the Lead Ii Nitrate And Sodium Hydroxide Balanced Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. aqueous solutions of lead (ii) nitrate and. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVEDPostlab Practice 1.0 &.) Write the balanced equation for the double replacement reaction Lead Ii Nitrate And Sodium Hydroxide Balanced Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). The products of the reaction are an aqueous solution of. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. How. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED 2.Aqueous solutions of aqueous lead(II) nitrate and sodium iodide are mixed. Show three Lead Ii Nitrate And Sodium Hydroxide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. The products of the reaction are an aqueous solution of. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. How do you write the balanced equation. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From mariabsmitho.blob.core.windows.net
Lead Hydroxide Reaction Equation at mariabsmitho blog Lead Ii Nitrate And Sodium Hydroxide Balanced Equation Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. enter an equation of an ionic chemical equation and press the balance button. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVEDIf 96.3 mL of lead(II) nitrate solution reacts completely with excess sodium iodide Lead Ii Nitrate And Sodium Hydroxide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. The products of the reaction are an aqueous solution of. enter an. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved 21. When lead (II) nitrate reacts with sodium iodide, Lead Ii Nitrate And Sodium Hydroxide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. How do you write the balanced equation. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. when aqueous sodium hydroxide is. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From fyoqdrqhk.blob.core.windows.net
Lead(Ii) Nitrate And Ammonium Chloride Net Ionic Equation at Pamela Armbruster blog Lead Ii Nitrate And Sodium Hydroxide Balanced Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons blog Lead Ii Nitrate And Sodium Hydroxide Balanced Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. enter an equation of an ionic chemical equation and press the balance button. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. The products of the reaction are an aqueous solution of.. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved 9. lead (II) nitrate + barium nitrate 10. Icad (II) Lead Ii Nitrate And Sodium Hydroxide Balanced Equation How do you write the balanced equation. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. The balanced equation will be calculated along. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. use the calculator below to balance chemical equations and determine the type. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From fyojqwyda.blob.core.windows.net
Lead Hydroxide Reaction at Jonathan Ertl blog Lead Ii Nitrate And Sodium Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. How do you write the balanced equation. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. use. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From kunduz.com
[ANSWERED] Write the balanced chemical equation for each of the reactions. Include phases. 1 Lead Ii Nitrate And Sodium Hydroxide Balanced Equation How do you write the balanced equation. enter an equation of an ionic chemical equation and press the balance button. use the calculator below to balance chemical equations and determine the type of reaction (instructions). when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. The products of. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.youtube.com
Double displacement Pb(NO3)2 + NaOH Lead(II) nitrate + Sodium hydroxide Precipitation Lead Ii Nitrate And Sodium Hydroxide Balanced Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. The products of the reaction are an aqueous solution of. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From fyoqdrqhk.blob.core.windows.net
Lead(Ii) Nitrate And Ammonium Chloride Net Ionic Equation at Pamela Armbruster blog Lead Ii Nitrate And Sodium Hydroxide Balanced Equation The balanced equation will be calculated along. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. How do you write the balanced equation. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. enter an equation of an ionic chemical equation and. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.youtube.com
Write the balanced chemical equation for each of the following reactions Lead nitrate is heated Lead Ii Nitrate And Sodium Hydroxide Balanced Equation The products of the reaction are an aqueous solution of. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. when carbon dioxide is dissolved in an aqueous solution of sodium. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.slideserve.com
PPT Chemical Reactions and Equations PowerPoint Presentation, free download ID9392686 Lead Ii Nitrate And Sodium Hydroxide Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVEDDoes a reaction occur when aqueous solutions of sodium sulfate and lead(II) nitrate are Lead Ii Nitrate And Sodium Hydroxide Balanced Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Lead Ii Nitrate And Sodium Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. The balanced equation will be calculated along. use the calculator below to balance chemical equations and determine the type of reaction (instructions). aqueous solutions. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.slideshare.net
Precipitation react2 Lead Ii Nitrate And Sodium Hydroxide Balanced Equation How do you write the balanced equation. use the calculator below to balance chemical equations and determine the type of reaction (instructions). aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. The balanced equation will be calculated along. The. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From fyocrjxru.blob.core.windows.net
Lead Nitrate And Calcium Iodide Balanced Equation at Michael Montagna blog Lead Ii Nitrate And Sodium Hydroxide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. use the calculator below to balance chemical equations and determine the type of reaction (instructions). when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Kbr (s) → k + (aq) + br. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Enter a balanced equation for the reaction between aqueous lead(II) nitrate and aqueous Lead Ii Nitrate And Sodium Hydroxide Balanced Equation How do you write the balanced equation. use the calculator below to balance chemical equations and determine the type of reaction (instructions). The products of the reaction are an aqueous solution of. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. Kbr (s) → k + (aq) + br − (aq) not. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead(II) nitrate and aqueous Lead Ii Nitrate And Sodium Hydroxide Balanced Equation when aqueous sodium hydroxide is added to a solution containing lead(ii) nitrate, a solid precipitate forms. How do you write the balanced equation. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. use the calculator. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.youtube.com
lead (II) nitrate And Sodium Iodide Make Lead (II) iodide And sodium Nitrate YouTube Lead Ii Nitrate And Sodium Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. How do you write the balanced equation. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. use the calculator below to balance chemical equations and determine the type of reaction (instructions). The products of the reaction are an. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double displacement reaction with Lead Ii Nitrate And Sodium Hydroxide Balanced Equation when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. How do you write the balanced equation. enter. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Na2S + Zn(NO3)2 = NaNO3 + ZnS YouTube Lead Ii Nitrate And Sodium Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From giovauxoc.blob.core.windows.net
Lead(Ii) Nitrate And Sodium Chloride Empirical Formula at Lillian Dietrich blog Lead Ii Nitrate And Sodium Hydroxide Balanced Equation The balanced equation will be calculated along. How do you write the balanced equation. The products of the reaction are an aqueous solution of. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go their own way, but the sulfate ion stays together as the sulfate ion. enter an equation of. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved An aqueous solution of lead(II) nitrate is mixed with Lead Ii Nitrate And Sodium Hydroxide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. use the calculator below to balance chemical equations and determine the type of reaction (instructions). The products of the reaction are an aqueous solution of. Kbr (s) → k + (aq) + br − (aq) not only do the two sodium ions go. Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVEDWhen a solution of lead(II) nitrate is mixed with a solution of sodium chromate, a yellow Lead Ii Nitrate And Sodium Hydroxide Balanced Equation enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. The products of the reaction are an aqueous solution of. when carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. How do you write the balanced equation. Kbr (s). Lead Ii Nitrate And Sodium Hydroxide Balanced Equation.