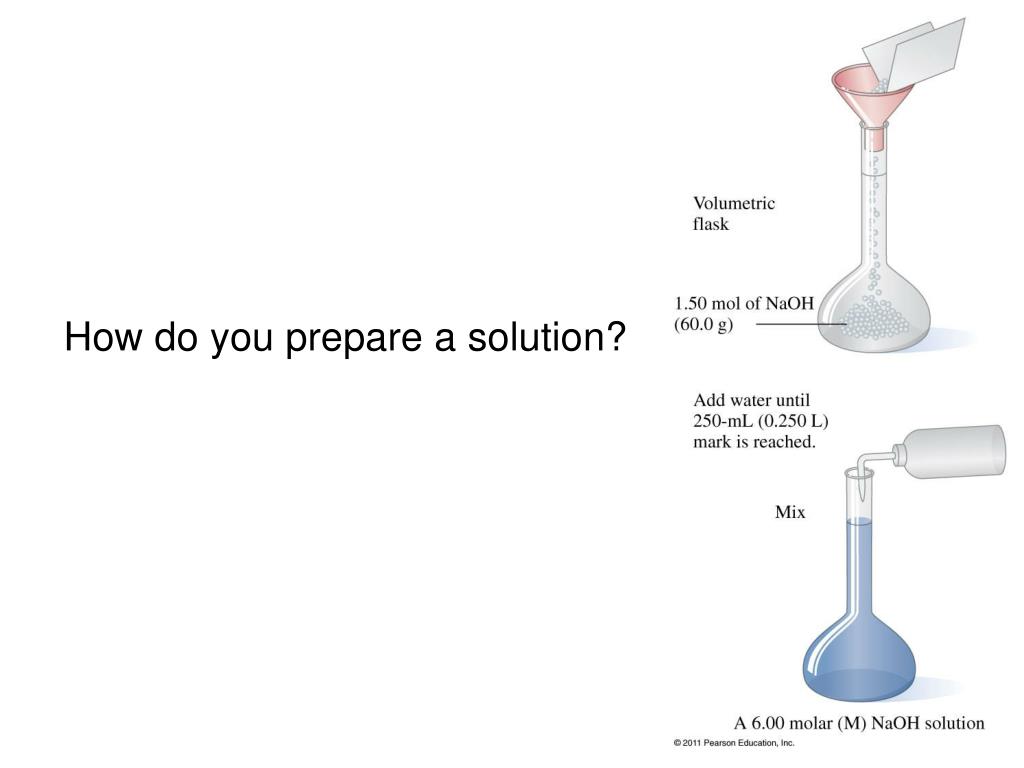
Solution Preparation Quizlet . Solutions cannot be separated by. (a) 500 ml of approximately 0.20 m naoh using solid naoh;. One of the most common ways to express the concentration of. To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. Distribution of particles in a solution is uniform 2. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. Components of a solution do not separate on standing 3. Describe how to prepare the following three solutions:
from www.slideserve.com
Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Describe how to prepare the following three solutions: (a) 500 ml of approximately 0.20 m naoh using solid naoh;. Components of a solution do not separate on standing 3. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. One of the most common ways to express the concentration of. To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. Distribution of particles in a solution is uniform 2.
PPT Chapter 14 PowerPoint Presentation, free download ID1459959
Solution Preparation Quizlet Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. Components of a solution do not separate on standing 3. (a) 500 ml of approximately 0.20 m naoh using solid naoh;. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. One of the most common ways to express the concentration of. Solutions cannot be separated by. Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. Describe how to prepare the following three solutions: Distribution of particles in a solution is uniform 2.
From studylib.net
Solution Preparation and Dilutions Solution Preparation Quizlet Describe how to prepare the following three solutions: Solutions cannot be separated by. (a) 500 ml of approximately 0.20 m naoh using solid naoh;. Components of a solution do not separate on standing 3. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Distribution of particles in a solution is uniform. Solution Preparation Quizlet.
From spmchemistry.blog.onlinetuition.com.my
Preparing Standard Solutions SPM Chemistry Solution Preparation Quizlet Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. Distribution of particles in a solution is uniform 2. Describe how to prepare the following three solutions: (a) 500. Solution Preparation Quizlet.
From www.pdfprof.com
isotonic solution definition quizlet Solution Preparation Quizlet Describe how to prepare the following three solutions: Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. Solutions cannot be separated by. Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. Components of a solution do not separate on. Solution Preparation Quizlet.
From www.techlearning.com
What is Quizlet and How Can I Teach With It? What's New? Tech & Learning Solution Preparation Quizlet To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. Components of a solution do not separate on standing 3. Distribution of particles in a solution is uniform 2. Describe how to prepare the following three. Solution Preparation Quizlet.
From chemistry98.blogspot.com
Chem Easy preparation standard solution Solution Preparation Quizlet You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a. Solution Preparation Quizlet.
From www.studocu.com
Chemical Solution Preparation Formulas 1107 Studocu Solution Preparation Quizlet Solutions cannot be separated by. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. One of the most common ways to express the concentration of. Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m.. Solution Preparation Quizlet.
From www.slideserve.com
PPT Experiment Solutions Preparation, Part 1 PowerPoint Presentation ID4107776 Solution Preparation Quizlet Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Components of a solution do not separate on standing 3. Which equation do we need to find out how many moles of nh4cl would there be. Solution Preparation Quizlet.
From quizlet.com
Show how the acetamidomalonate method can be used to prepare Quizlet Solution Preparation Quizlet (a) 500 ml of approximately 0.20 m naoh using solid naoh;. Components of a solution do not separate on standing 3. Solutions cannot be separated by. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. Which equation do we need to find out how many moles of nh4cl. Solution Preparation Quizlet.
From www.slideserve.com
PPT Chapter 3—Stoichiometry of Formulas and Equations PowerPoint Presentation ID583497 Solution Preparation Quizlet Components of a solution do not separate on standing 3. Describe how to prepare the following three solutions: Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. Solutions cannot be separated by. Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a. Solution Preparation Quizlet.
From www.slideserve.com
PPT Solution Preparation and Dilutions PowerPoint Presentation, free download ID8907626 Solution Preparation Quizlet Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of. Solution Preparation Quizlet.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Solution Preparation Quizlet Components of a solution do not separate on standing 3. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. Solutions cannot be separated by. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. To prepare a solution that contains. Solution Preparation Quizlet.
From www.chegg.com
Solved molarity, dilutions, and preparing solutions lab Solution Preparation Quizlet Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. Components of a solution do not separate on standing 3. Distribution of particles in a solution is uniform 2. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout.. Solution Preparation Quizlet.
From www.youtube.com
How To Prepare Solutions YouTube Solution Preparation Quizlet One of the most common ways to express the concentration of. Describe how to prepare the following three solutions: (a) 500 ml of approximately 0.20 m naoh using solid naoh;. Distribution of particles in a solution is uniform 2. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. You prepare a. Solution Preparation Quizlet.
From slideplayer.com
I. The Nature of Solutions ppt download Solution Preparation Quizlet (a) 500 ml of approximately 0.20 m naoh using solid naoh;. One of the most common ways to express the concentration of. Describe how to prepare the following three solutions: To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. A solution is a homogeneous mixture of two or more substances where. Solution Preparation Quizlet.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory, and Dilution Formulas Solution Preparation Quizlet Describe how to prepare the following three solutions: To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. Solutions cannot be separated by. (a) 500 ml of approximately 0.20 m naoh using solid naoh;. Components of. Solution Preparation Quizlet.
From quizlet.com
Prepare Working Solutions Flashcards Quizlet Solution Preparation Quizlet Describe how to prepare the following three solutions: Solutions cannot be separated by. Distribution of particles in a solution is uniform 2. Components of a solution do not separate on standing 3. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. One of the most common ways to express the concentration. Solution Preparation Quizlet.
From www.slideshare.net
Solutions preparation Solution Preparation Quizlet Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. Solutions cannot be. Solution Preparation Quizlet.
From www.youtube.com
Solution Preparation What is a standard solution? YouTube Solution Preparation Quizlet To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. Solutions cannot be separated by. Describe how to prepare the following three solutions: Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. Study with quizlet and memorize flashcards. Solution Preparation Quizlet.
From quizizz.com
Solution preparation 3.1.0.8 Quizizz Solution Preparation Quizlet Components of a solution do not separate on standing 3. (a) 500 ml of approximately 0.20 m naoh using solid naoh;. Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. A solution. Solution Preparation Quizlet.
From scicord.com
Solution Preparation SciCord, LLC Solution Preparation Quizlet Components of a solution do not separate on standing 3. Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. One of the most common ways to express the concentration of. Describe how to prepare the following three solutions: Distribution of particles in a solution is uniform 2. Solutions cannot be separated by. You. Solution Preparation Quizlet.
From www.slideshare.net
Solutions preparation Solution Preparation Quizlet Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Components of a solution do not separate on standing 3. Solutions cannot be separated by. (a) 500 ml of approximately 0.20 m naoh using solid naoh;.. Solution Preparation Quizlet.
From quizlet.com
Santana Rey, owner of Business Solutions, decides to prepare Quizlet Solution Preparation Quizlet Distribution of particles in a solution is uniform 2. (a) 500 ml of approximately 0.20 m naoh using solid naoh;. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Which. Solution Preparation Quizlet.
From ashtynldherring.blogspot.com
Preparation of Standard Solution AshtynldHerring Solution Preparation Quizlet Solutions cannot be separated by. Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. Distribution of particles in a solution is uniform 2. You prepare a solution by dissolving a. Solution Preparation Quizlet.
From www.slideshare.net
Solutions preparation Solution Preparation Quizlet Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. Distribution of particles in a solution is uniform 2. Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. A solution is a homogeneous mixture of two or more substances where. Solution Preparation Quizlet.
From labpedia.net
Solutions Part 2 Preparation of Solutions (Molar, Normal, and Dilution) Solution Preparation Quizlet Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. Describe how to prepare the following three solutions: Distribution of particles in a solution is uniform 2. One of the most common ways to express the concentration of. A solution is a homogeneous mixture of two or more substances where the composition is the. Solution Preparation Quizlet.
From quizlet.com
textbf { Prepare Quizlet Solution Preparation Quizlet (a) 500 ml of approximately 0.20 m naoh using solid naoh;. Components of a solution do not separate on standing 3. To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Study with quizlet. Solution Preparation Quizlet.
From www.slideshare.net
Chemical Laboratory Solution Preparation Presentation PPT Solution Preparation Quizlet One of the most common ways to express the concentration of. A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Describe how to prepare the following three solutions: Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. Distribution of particles in a solution is. Solution Preparation Quizlet.
From www.slideshare.net
Solutions preparation Solution Preparation Quizlet A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. Describe how to prepare the following three solutions: Solutions cannot be separated by. Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. One of the most common ways to express the concentration of. (a) 500. Solution Preparation Quizlet.
From www.studypool.com
SOLUTION Labster Solution Preparation From Salt to Solution Laboratory Report Studypool Solution Preparation Quizlet (a) 500 ml of approximately 0.20 m naoh using solid naoh;. Components of a solution do not separate on standing 3. Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. One of the most common ways to express the concentration of. Solutions cannot be separated by. Describe how to prepare the following three. Solution Preparation Quizlet.
From quizlet.com
Prepare a flowchart for the design of doubly reinforced rect Quizlet Solution Preparation Quizlet A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. Describe how to prepare the following three solutions: Components of a solution do not separate on standing 3. One of the most common ways. Solution Preparation Quizlet.
From www.studypool.com
SOLUTION Chem120 Week 3 Solution Preparation From Salt to Solution Lab 6 Studypool Solution Preparation Quizlet (a) 500 ml of approximately 0.20 m naoh using solid naoh;. Components of a solution do not separate on standing 3. Solutions cannot be separated by. Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. Study with quizlet and memorize flashcards containing terms like solution preparation,. Solution Preparation Quizlet.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID1459959 Solution Preparation Quizlet Which equation do we need to find out how many moles of nh4cl would there be in 500 ml of a 0.300 m. Describe how to prepare the following three solutions: You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. Solutions cannot be separated by. A solution is. Solution Preparation Quizlet.
From www.studypool.com
SOLUTION Chem120 Week 3 Solution Preparation From Salt to Solution Lab 6 Studypool Solution Preparation Quizlet Study with quizlet and memorize flashcards containing terms like solution preparation, solution concentration, concentration and more. Distribution of particles in a solution is uniform 2. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. Describe how to prepare the following three solutions: Components of a solution do not. Solution Preparation Quizlet.
From quizlet.com
Week 6 Laboratory Setup Reagent Preparation Room Diagram Quizlet Solution Preparation Quizlet Distribution of particles in a solution is uniform 2. (a) 500 ml of approximately 0.20 m naoh using solid naoh;. Solutions cannot be separated by. One of the most common ways to express the concentration of. Describe how to prepare the following three solutions: Which equation do we need to find out how many moles of nh4cl would there be. Solution Preparation Quizlet.
From www.youtube.com
Préparation d'une solution par dissolution (Exemple d'exercice avec sa résolution, module 3.5 Solution Preparation Quizlet A solution is a homogeneous mixture of two or more substances where the composition is the same throughout. To prepare a solution that contains a specified concentration of a substance, it is necessary to dissolve the. You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. Study with quizlet. Solution Preparation Quizlet.