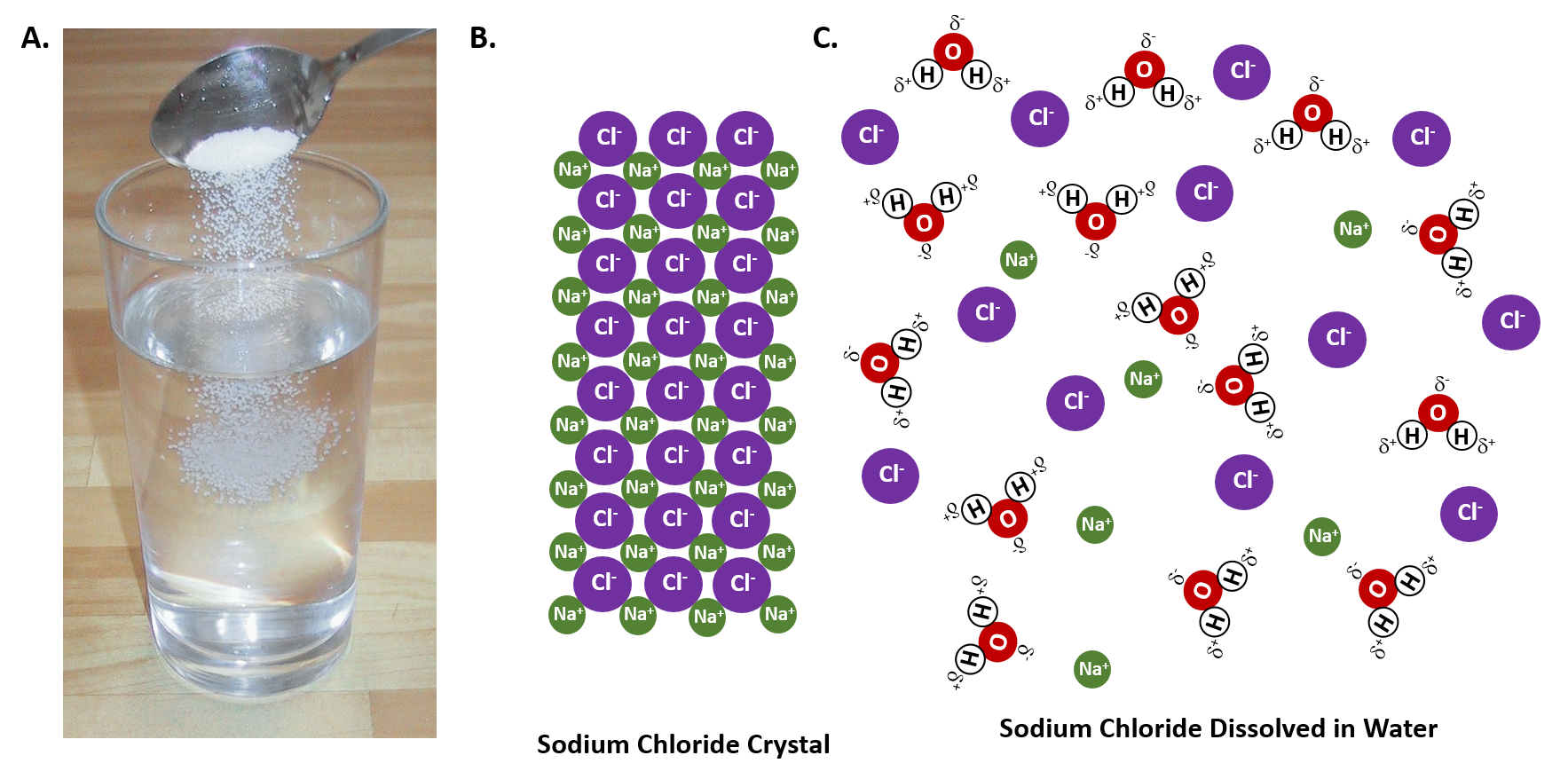
Why Does Salt Crystals Dissolve In Water . A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water When salt is added to water, the positive and negative ions separate. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The positive areas of the water molecules surround the negative chloride ions. The negative areas of the water molecules surround the. The water molecules surround the ions and pull them. Why dissolving salt is a chemical change.
from wou.edu
The water molecules surround the ions and pull them. The negative areas of the water molecules surround the. When salt is added to water, the positive and negative ions separate. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Why dissolving salt is a chemical change. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The positive areas of the water molecules surround the negative chloride ions.
CH104 Chapter 7 Solutions Chemistry
Why Does Salt Crystals Dissolve In Water The negative areas of the water molecules surround the. The positive areas of the water molecules surround the negative chloride ions. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Why dissolving salt is a chemical change. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The negative areas of the water molecules surround the. When salt is added to water, the positive and negative ions separate. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The water molecules surround the ions and pull them.
From www.youtube.com
Why Salt Dissolve In Water/உப்பு ஏன் தண்ணீரில் கரைகிறது YouTube Why Does Salt Crystals Dissolve In Water Why dissolving salt is a chemical change. The water molecules surround the ions and pull them. The positive areas of the water molecules surround the negative chloride ions. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The negative areas of the water molecules surround the. Water molecules. Why Does Salt Crystals Dissolve In Water.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? Why Does Salt Crystals Dissolve In Water Why dissolving salt is a chemical change. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The water molecules surround the ions and pull them. The negative. Why Does Salt Crystals Dissolve In Water.
From mungfali.com
Dissolving Salt In Water Diagram Why Does Salt Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The water molecules surround the ions and pull them. When salt is added to water, the positive and negative ions separate. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The negative areas. Why Does Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID2736092 Why Does Salt Crystals Dissolve In Water Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The negative areas of the water molecules surround the. The water molecules surround the ions and pull them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The positive areas of the water. Why Does Salt Crystals Dissolve In Water.
From butjustwhy.com
Why does salt dissolve in water? But Just Why!? Why Does Salt Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The negative areas of the water molecules surround the. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Why dissolving salt is a chemical change. The water. Why Does Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID6136171 Why Does Salt Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride). Why Does Salt Crystals Dissolve In Water.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Does Salt Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to. Why Does Salt Crystals Dissolve In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Why Does Salt Crystals Dissolve In Water Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The negative areas of the water molecules surround the. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water When salt is added to water, the positive and negative ions separate. The water molecules. Why Does Salt Crystals Dissolve In Water.
From www.youtube.com
Why Salt (NaCl) dissolve in water YouTube Why Does Salt Crystals Dissolve In Water Why dissolving salt is a chemical change. The water molecules surround the ions and pull them. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water We will first examine the process that occurs. Why Does Salt Crystals Dissolve In Water.
From lifeboat.com
We finally know in detail how salt dissolves in water Why Does Salt Crystals Dissolve In Water The water molecules surround the ions and pull them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water When salt is added to water, the positive and negative ions separate. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. We will first. Why Does Salt Crystals Dissolve In Water.
From exyjtpwfh.blob.core.windows.net
Why Do Sodium Chloride Crystals Dissolve In Water at Tracy White blog Why Does Salt Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The water molecules surround the ions and pull them. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The negative areas of the water molecules surround the. Why dissolving salt is a chemical. Why Does Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID4324596 Why Does Salt Crystals Dissolve In Water When salt is added to water, the positive and negative ions separate. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The positive areas of the water molecules surround the negative chloride ions. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then. Why Does Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID4324596 Why Does Salt Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Why dissolving salt is a chemical change. The negative areas of the water molecules surround the. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. We will first examine the process that occurs. Why Does Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free download ID3057625 Why Does Salt Crystals Dissolve In Water The water molecules surround the ions and pull them. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Water molecules pulling apart the ions (sodium and chloride). Why Does Salt Crystals Dissolve In Water.
From curiosityguide.org
How Does Salt Dissolve in Water? Curiosity Guide Why Does Salt Crystals Dissolve In Water When salt is added to water, the positive and negative ions separate. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The positive areas of the water molecules surround the negative chloride ions.. Why Does Salt Crystals Dissolve In Water.
From www.youtube.com
Salt dissolves in water Is this a chemical or physical change? YouTube Why Does Salt Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The water molecules surround the ions and pull them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The positive areas of the water molecules surround the. Why Does Salt Crystals Dissolve In Water.
From courses.lumenlearning.com
Water Introduction to Biology Why Does Salt Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Why dissolving salt is a chemical change. When salt is added to water, the positive and negative ions. Why Does Salt Crystals Dissolve In Water.
From www.youtube.com
How Salt Dissolves in Water? YouTube Why Does Salt Crystals Dissolve In Water The water molecules surround the ions and pull them. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. A machine learning model has revealed how crystals of. Why Does Salt Crystals Dissolve In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Art of Science Why Does Salt Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The negative areas of the water molecules surround the. The positive areas of the water molecules surround the negative chloride ions. The water molecules surround the ions and pull them. Water molecules pulling apart the ions (sodium and chloride). Why Does Salt Crystals Dissolve In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Art of Science Why Does Salt Crystals Dissolve In Water When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. The positive areas of the water molecules surround the negative chloride ions. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water molecules pulling apart the ions. Why Does Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 Why Does Salt Crystals Dissolve In Water We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The water molecules surround the ions and pull them. Water molecules pulling apart the ions (sodium and chloride). Why Does Salt Crystals Dissolve In Water.
From studylibstearine.z21.web.core.windows.net
How Does Water Dissolve Salt Why Does Salt Crystals Dissolve In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The water molecules surround the ions and pull them. When salt is added to water, the positive and negative ions separate. Why dissolving salt is a chemical change. The positive areas of the water molecules surround the negative chloride. Why Does Salt Crystals Dissolve In Water.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Does Salt Crystals Dissolve In Water The negative areas of the water molecules surround the. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The water molecules surround the ions and pull them. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. A machine learning model has revealed. Why Does Salt Crystals Dissolve In Water.
From www.labkafe.com
Solubility of Salts ‒ Why Common Salts are So Soluble in Water Labkafe Why Does Salt Crystals Dissolve In Water When salt is added to water, the positive and negative ions separate. The negative areas of the water molecules surround the. Why dissolving salt is a chemical change. The positive areas of the water molecules surround the negative chloride ions. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in. Why Does Salt Crystals Dissolve In Water.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Why Does Salt Crystals Dissolve In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The positive areas of the water molecules surround the negative chloride ions. We will first examine the process. Why Does Salt Crystals Dissolve In Water.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Does Salt Crystals Dissolve In Water The negative areas of the water molecules surround the. The water molecules surround the ions and pull them. The positive areas of the water molecules surround the negative chloride ions. Why dissolving salt is a chemical change. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. We will. Why Does Salt Crystals Dissolve In Water.
From bettertogether.org
Is Salt Dissolving In Water A Chemical Change Better Together Why Does Salt Crystals Dissolve In Water Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. Why dissolving salt is a chemical change. When salt is added to water, the positive and negative ions separate. The negative areas of the water molecules surround the. We will first examine the process that occurs when an ionic compound such as table salt (sodium. Why Does Salt Crystals Dissolve In Water.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Does Salt Crystals Dissolve In Water The positive areas of the water molecules surround the negative chloride ions. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The negative areas of the water molecules surround the. The water molecules. Why Does Salt Crystals Dissolve In Water.
From mungfali.com
Dissolving Salt In Water Diagram Why Does Salt Crystals Dissolve In Water Why dissolving salt is a chemical change. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. When salt is added to water, the positive and negative ions separate. The negative areas of the water molecules surround the. Water molecules pulling apart the ions (sodium and chloride) in a. Why Does Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Do Now PowerPoint Presentation, free download ID5743791 Why Does Salt Crystals Dissolve In Water The negative areas of the water molecules surround the. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The positive areas of the water molecules surround the negative chloride ions. When salt is added to water, the positive and negative ions separate. Water molecules pulling apart the ions. Why Does Salt Crystals Dissolve In Water.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Visionlearning Why Does Salt Crystals Dissolve In Water Why dissolving salt is a chemical change. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The positive areas of the water molecules surround the negative chloride ions. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The negative areas of the. Why Does Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free download ID1242210 Why Does Salt Crystals Dissolve In Water The water molecules surround the ions and pull them. Why dissolving salt is a chemical change. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water We will. Why Does Salt Crystals Dissolve In Water.
From manualwiringbrancher.z14.web.core.windows.net
Diagram Of Salt Dissolving In Water Why Does Salt Crystals Dissolve In Water The water molecules surround the ions and pull them. When salt is added to water, the positive and negative ions separate. The negative areas of the water molecules surround the. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The positive areas of the water molecules surround the. Why Does Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT SPONCH PowerPoint Presentation, free download ID556676 Why Does Salt Crystals Dissolve In Water When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. The negative areas of the water molecules surround the. Water molecules pulling apart the ions (sodium and chloride). Why Does Salt Crystals Dissolve In Water.
From webmis.highland.cc.il.us
Aqueous Solutions Why Does Salt Crystals Dissolve In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water When salt is added to water, the positive and negative ions separate. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Water molecules pulling apart the ions. Why Does Salt Crystals Dissolve In Water.