Iron Color Reaction . Rust requires three chemicals in order to form: In these cases, it simply acts as a base. Reactions of iron ions with ammonia. Ammonia can act as both a base and a ligand. In order to return to its ground state, the electron releases the additional energy. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Iron metal reacts with air, water vapor,. Iron + water + oxygen → hydrated iron (iii) oxide. Reactions of the iron ions with ammonia solution. This reaction excites an electron in the metal from its ground state to a higher orbital. Ammonia can act as both a base and a ligand. This is an example of an electrochemical reaction and.
from www.slideserve.com
Iron metal reacts with air, water vapor,. In order to return to its ground state, the electron releases the additional energy. Iron + water + oxygen → hydrated iron (iii) oxide. In these cases, it simply acts as a base. Ammonia can act as both a base and a ligand. This reaction excites an electron in the metal from its ground state to a higher orbital. Reactions of iron ions with ammonia. Reactions of the iron ions with ammonia solution. This is an example of an electrochemical reaction and. Ammonia can act as both a base and a ligand.
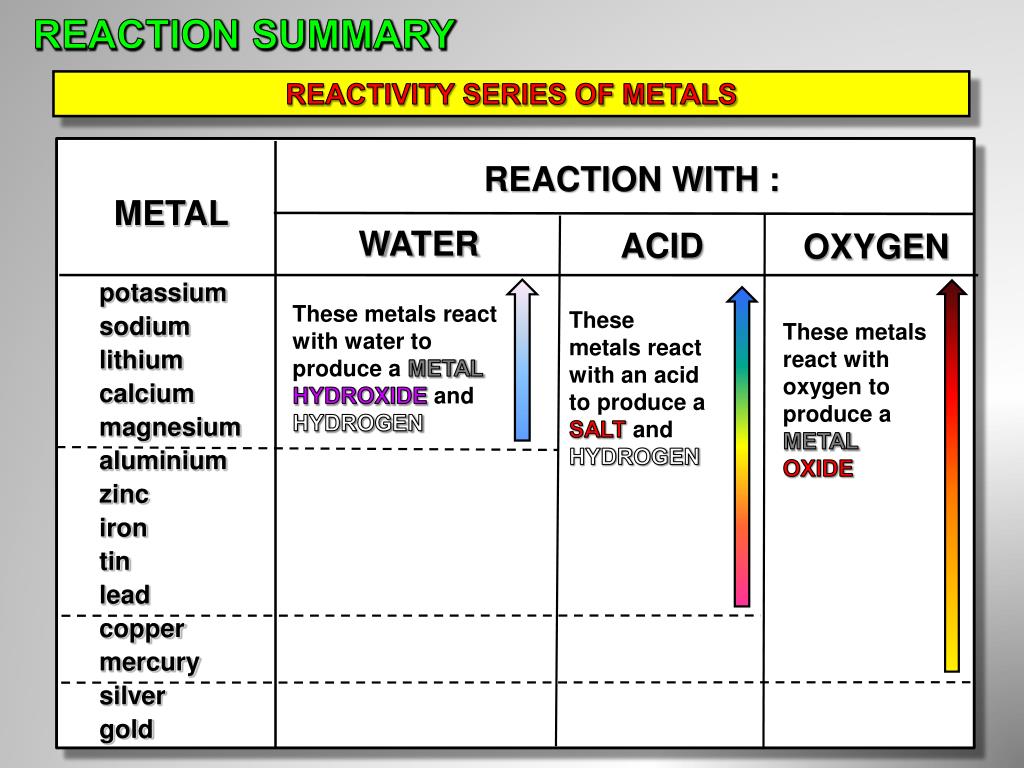
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563
Iron Color Reaction Reactions of iron ions with ammonia. In these cases, it simply acts as a base. Ammonia can act as both a base and a ligand. Ammonia can act as both a base and a ligand. Reactions of the iron ions with ammonia solution. This is an example of an electrochemical reaction and. Iron metal reacts with air, water vapor,. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Rust requires three chemicals in order to form: This reaction excites an electron in the metal from its ground state to a higher orbital. Reactions of iron ions with ammonia. In order to return to its ground state, the electron releases the additional energy. Iron + water + oxygen → hydrated iron (iii) oxide.
From www.slideserve.com
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563 Iron Color Reaction Reactions of iron ions with ammonia. Rust requires three chemicals in order to form: This is an example of an electrochemical reaction and. This reaction excites an electron in the metal from its ground state to a higher orbital. Iron + water + oxygen → hydrated iron (iii) oxide. Common chemical reactions of iron relate to iron in either a. Iron Color Reaction.
From www.expii.com
Color and Oxidation State — Overview & Examples Expii Iron Color Reaction Reactions of the iron ions with ammonia solution. Rust requires three chemicals in order to form: This is an example of an electrochemical reaction and. Iron + water + oxygen → hydrated iron (iii) oxide. This reaction excites an electron in the metal from its ground state to a higher orbital. Reactions of iron ions with ammonia. Ammonia can act. Iron Color Reaction.
From www.pinterest.com
Lysine Iron Agar is used to distinguish between bacterial pathogens Iron Color Reaction Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. This is an example of an electrochemical reaction and. Ammonia can act as both a base and a ligand. Iron + water + oxygen → hydrated iron (iii) oxide. In order to return to its ground state, the electron releases the additional energy. Reactions. Iron Color Reaction.
From www.yubrain.com
The flame color test Iron Color Reaction Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. In these cases, it simply acts as a base. This reaction excites an electron in the metal from its ground state to a higher orbital. Iron + water + oxygen → hydrated iron (iii) oxide. Ammonia can act as both a base and a. Iron Color Reaction.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Iron Color Reaction Reactions of the iron ions with ammonia solution. In these cases, it simply acts as a base. Reactions of iron ions with ammonia. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Ammonia can act as both a base and a ligand. In order to return to its ground state, the electron releases. Iron Color Reaction.
From ca.ping.com
PING Iron Color Code Chart Iron Color Reaction Iron + water + oxygen → hydrated iron (iii) oxide. In these cases, it simply acts as a base. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Ammonia can act as both a base and a ligand. Iron metal reacts with air, water vapor,. Reactions of iron ions with ammonia. Ammonia can. Iron Color Reaction.
From www.alamy.com
Deformed rusted iron texture with blos and dents. With green and orange Iron Color Reaction Ammonia can act as both a base and a ligand. This reaction excites an electron in the metal from its ground state to a higher orbital. This is an example of an electrochemical reaction and. In order to return to its ground state, the electron releases the additional energy. Ammonia can act as both a base and a ligand. Iron. Iron Color Reaction.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Iron Color Reaction Reactions of the iron ions with ammonia solution. This is an example of an electrochemical reaction and. This reaction excites an electron in the metal from its ground state to a higher orbital. Iron metal reacts with air, water vapor,. Reactions of iron ions with ammonia. In these cases, it simply acts as a base. Ammonia can act as both. Iron Color Reaction.
From colorscombo.com
What Color Is Iron Iron Color Reaction This is an example of an electrochemical reaction and. Reactions of the iron ions with ammonia solution. In order to return to its ground state, the electron releases the additional energy. In these cases, it simply acts as a base. This reaction excites an electron in the metal from its ground state to a higher orbital. Iron metal reacts with. Iron Color Reaction.
From homepage.ufp.pt
Pagina F (Termos) Iron Color Reaction Iron metal reacts with air, water vapor,. Ammonia can act as both a base and a ligand. Ammonia can act as both a base and a ligand. In order to return to its ground state, the electron releases the additional energy. Iron + water + oxygen → hydrated iron (iii) oxide. This is an example of an electrochemical reaction and.. Iron Color Reaction.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Iron Color Reaction In these cases, it simply acts as a base. Ammonia can act as both a base and a ligand. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Reactions of the iron ions with ammonia solution. Ammonia can act as both a base and a ligand. This is an example of an electrochemical. Iron Color Reaction.
From chemistry.com.pk
Metal Ion Flame Test Colours [Infographic] Iron Color Reaction Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Ammonia can act as both a base and a ligand. In these cases, it simply acts as a base. Reactions of iron ions with ammonia. In order to return to its ground state, the electron releases the additional energy. This reaction excites an electron. Iron Color Reaction.
From lessonlibminimalist.z13.web.core.windows.net
Chemical Reaction Science Project Iron Color Reaction In these cases, it simply acts as a base. Ammonia can act as both a base and a ligand. Reactions of the iron ions with ammonia solution. Ammonia can act as both a base and a ligand. In order to return to its ground state, the electron releases the additional energy. Common chemical reactions of iron relate to iron in. Iron Color Reaction.
From www.revisechemistry.uk
Identifying the products of chemical reactions (chemistry only) OCR Iron Color Reaction Rust requires three chemicals in order to form: Ammonia can act as both a base and a ligand. This is an example of an electrochemical reaction and. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Iron + water + oxygen → hydrated iron (iii) oxide. Iron metal reacts with air, water vapor,.. Iron Color Reaction.
From askfilo.com
17 Why does the colour of copper sulphate solution change when an iron na.. Iron Color Reaction In order to return to its ground state, the electron releases the additional energy. Rust requires three chemicals in order to form: Iron metal reacts with air, water vapor,. Reactions of iron ions with ammonia. Ammonia can act as both a base and a ligand. This reaction excites an electron in the metal from its ground state to a higher. Iron Color Reaction.
From www.etsy.com
Iron Ore Paint Color Palette With Coordinating Colors to Use Etsy Ireland Iron Color Reaction Reactions of the iron ions with ammonia solution. This reaction excites an electron in the metal from its ground state to a higher orbital. Iron + water + oxygen → hydrated iron (iii) oxide. Ammonia can act as both a base and a ligand. In order to return to its ground state, the electron releases the additional energy. In these. Iron Color Reaction.
From www.researchgate.net
Scheme 1. The reactions involved in the production of iron oxide Iron Color Reaction This reaction excites an electron in the metal from its ground state to a higher orbital. Ammonia can act as both a base and a ligand. This is an example of an electrochemical reaction and. Reactions of iron ions with ammonia. Iron metal reacts with air, water vapor,. In these cases, it simply acts as a base. Rust requires three. Iron Color Reaction.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) YouTube Iron Color Reaction Reactions of iron ions with ammonia. Iron metal reacts with air, water vapor,. Iron + water + oxygen → hydrated iron (iii) oxide. In these cases, it simply acts as a base. Reactions of the iron ions with ammonia solution. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. This is an example. Iron Color Reaction.
From www.scienceabc.com
Single Replacement Reaction Vs Double Replacement Reaction Differences Iron Color Reaction Ammonia can act as both a base and a ligand. In these cases, it simply acts as a base. In order to return to its ground state, the electron releases the additional energy. Iron metal reacts with air, water vapor,. Rust requires three chemicals in order to form: Reactions of the iron ions with ammonia solution. Ammonia can act as. Iron Color Reaction.
From mammothmemory.net
When metals are heated it reacts with oxygen to create flame Iron Color Reaction Rust requires three chemicals in order to form: Reactions of the iron ions with ammonia solution. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Reactions of iron ions with ammonia. Ammonia can act as both a base and a ligand. This reaction excites an electron in the metal from its ground state. Iron Color Reaction.
From animalia-life.club
Iron Sulfate Solution Coloring Pages Iron Color Reaction Ammonia can act as both a base and a ligand. This is an example of an electrochemical reaction and. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Rust requires three chemicals in order to form: Reactions of the iron ions with ammonia solution. Iron + water + oxygen → hydrated iron (iii). Iron Color Reaction.
From www.youtube.com
Reactions of Iron Reactions Chemistry FuseSchool YouTube Iron Color Reaction Iron + water + oxygen → hydrated iron (iii) oxide. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Iron metal reacts with air, water vapor,. In order to return to its ground state, the electron releases the additional energy. This is an example of an electrochemical reaction and. Reactions of iron ions. Iron Color Reaction.
From www.reddit.com
Precipitate Colors of Metal Ions in Aqueous Ammonia and Sodium Iron Color Reaction In these cases, it simply acts as a base. Ammonia can act as both a base and a ligand. This reaction excites an electron in the metal from its ground state to a higher orbital. Iron metal reacts with air, water vapor,. Reactions of the iron ions with ammonia solution. This is an example of an electrochemical reaction and. In. Iron Color Reaction.
From www.dreamstime.com
A Table of Iron Compounds Colors Stock Vector Illustration Iron Color Reaction Reactions of iron ions with ammonia. In these cases, it simply acts as a base. Rust requires three chemicals in order to form: Ammonia can act as both a base and a ligand. Iron metal reacts with air, water vapor,. Ammonia can act as both a base and a ligand. This is an example of an electrochemical reaction and. Iron. Iron Color Reaction.
From www.tessshebaylo.com
Hydrogen Peroxide And Iron 3 Chloride Balanced Equation Tessshebaylo Iron Color Reaction This is an example of an electrochemical reaction and. Ammonia can act as both a base and a ligand. Reactions of iron ions with ammonia. In these cases, it simply acts as a base. Iron + water + oxygen → hydrated iron (iii) oxide. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state.. Iron Color Reaction.
From www.youtube.com
Reaction of metals with water Class 10 Chemistry ICSE Board Iron Color Reaction Reactions of the iron ions with ammonia solution. Reactions of iron ions with ammonia. Ammonia can act as both a base and a ligand. Rust requires three chemicals in order to form: In order to return to its ground state, the electron releases the additional energy. In these cases, it simply acts as a base. Common chemical reactions of iron. Iron Color Reaction.
From www.facebook.com
Iron beast in diff... Alejandro’s Discounted Game Credits Iron Color Reaction Iron + water + oxygen → hydrated iron (iii) oxide. Rust requires three chemicals in order to form: Reactions of the iron ions with ammonia solution. This reaction excites an electron in the metal from its ground state to a higher orbital. This is an example of an electrochemical reaction and. Ammonia can act as both a base and a. Iron Color Reaction.
From sciencenotes.org
Transition Metal Ion Colors Iron Color Reaction Reactions of the iron ions with ammonia solution. In order to return to its ground state, the electron releases the additional energy. Ammonia can act as both a base and a ligand. This is an example of an electrochemical reaction and. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Reactions of iron. Iron Color Reaction.
From www.facebook.com
Iron beast in diff... Alejandro’s Discounted Game Credits Iron Color Reaction Ammonia can act as both a base and a ligand. In these cases, it simply acts as a base. This is an example of an electrochemical reaction and. Reactions of the iron ions with ammonia solution. This reaction excites an electron in the metal from its ground state to a higher orbital. Reactions of iron ions with ammonia. Rust requires. Iron Color Reaction.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记6.3.3 Reactions of Ions in Aqueous Iron Color Reaction This reaction excites an electron in the metal from its ground state to a higher orbital. In these cases, it simply acts as a base. In order to return to its ground state, the electron releases the additional energy. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. This is an example of. Iron Color Reaction.
From www.hotzxgirl.com
Triple Sugar Iron Agar Tsi Principle Procedure And Interpretation Hot Iron Color Reaction Reactions of iron ions with ammonia. Iron + water + oxygen → hydrated iron (iii) oxide. In order to return to its ground state, the electron releases the additional energy. Reactions of the iron ions with ammonia solution. This reaction excites an electron in the metal from its ground state to a higher orbital. In these cases, it simply acts. Iron Color Reaction.
From mostaql.com
Chemistry chart for irons reactions مستقل Iron Color Reaction Iron metal reacts with air, water vapor,. This reaction excites an electron in the metal from its ground state to a higher orbital. In these cases, it simply acts as a base. This is an example of an electrochemical reaction and. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Ammonia can act. Iron Color Reaction.
From sciencenotes.org
Fake Blood Chemical Reaction 3 Chemistry Demonstrations Iron Color Reaction Iron + water + oxygen → hydrated iron (iii) oxide. Rust requires three chemicals in order to form: This reaction excites an electron in the metal from its ground state to a higher orbital. In order to return to its ground state, the electron releases the additional energy. Ammonia can act as both a base and a ligand. Common chemical. Iron Color Reaction.
From microbiologylabnotes.blogspot.com
TRIPLE SUGAR IRON (TSI AGAR) Microbiology Lab Notes Iron Color Reaction Iron metal reacts with air, water vapor,. This is an example of an electrochemical reaction and. Common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Ammonia can act as both a base and a ligand. Rust requires three chemicals in order to form: Reactions of iron ions with ammonia. Ammonia can act as. Iron Color Reaction.
From chemistry.com.pk
Colours of Transition Metal Ions in Aqueous Solution [Infographic Iron Color Reaction This reaction excites an electron in the metal from its ground state to a higher orbital. Reactions of iron ions with ammonia. Iron + water + oxygen → hydrated iron (iii) oxide. Ammonia can act as both a base and a ligand. Rust requires three chemicals in order to form: Ammonia can act as both a base and a ligand.. Iron Color Reaction.