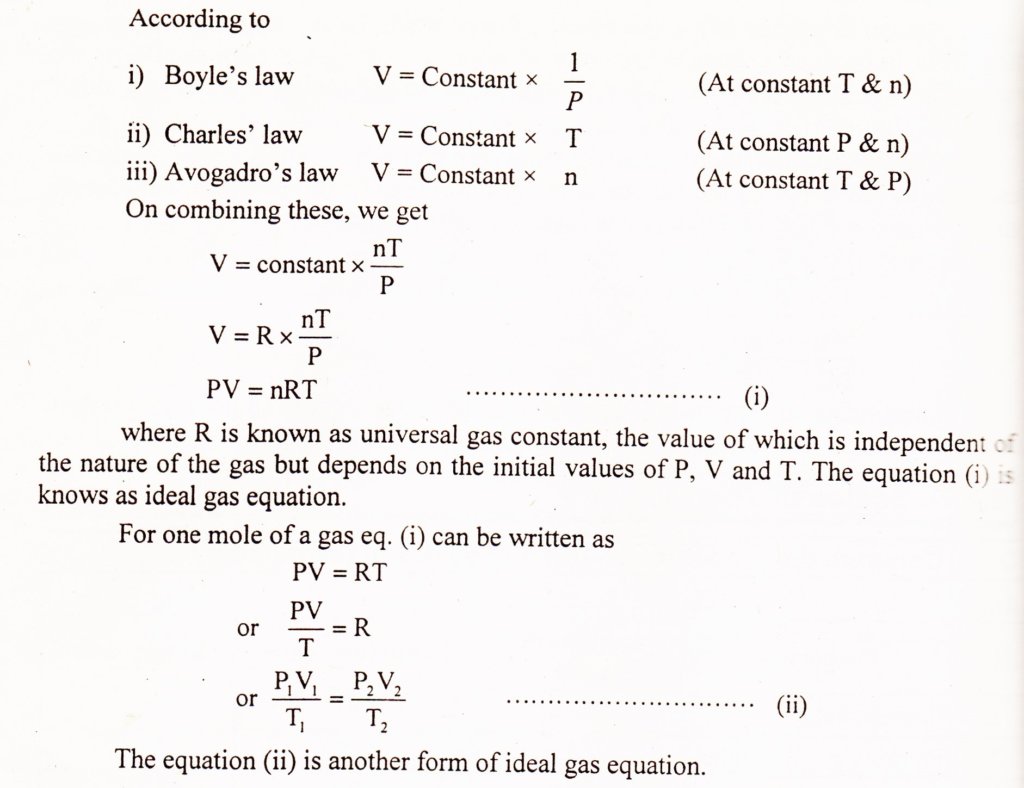
How To Derive Ideal Gas Law . (a) when air is pumped into a deflated tire, its volume first increases without much increase in pressure. What follows is just one way to derive the ideal gas law. Where, p is the pressure of the ideal gas. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. The ideal gas law was first written in 1834 by emil clapeyron. From charles’ law, v ∝ t. From boyle’s law, p ∝ 1/v. For a static sample of gas, we can write each of the six gas laws as follows: V is the volume of the ideal gas. This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. Suppose a gas consisting of n moles is at a pressure p, volume v, and temperature t. It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. Boyle’s law, charles’ law, and avogadro’s law. (b) when the tire is. The ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so.
from chemistryskills.com
Suppose a gas consisting of n moles is at a pressure p, volume v, and temperature t. The ideal gas law is the equation of state of a hypothetical ideal gas. For a static sample of gas, we can write each of the six gas laws as follows: This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. To derive the ideal gas equation, we combine the three basic gas laws: Where, p is the pressure of the ideal gas. V is the volume of the ideal gas. (b) when the tire is. From boyle’s law, p ∝ 1/v. The ideal gas equation can be written as.
Ideal Gas Law Combined Gas Law Chemistry Skills
How To Derive Ideal Gas Law From charles’ law, v ∝ t. (a) when air is pumped into a deflated tire, its volume first increases without much increase in pressure. What follows is just one way to derive the ideal gas law. V is the volume of the ideal gas. The ideal gas law is the equation of state of a hypothetical ideal gas. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. (b) when the tire is. It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. From boyle’s law, p ∝ 1/v. Suppose a gas consisting of n moles is at a pressure p, volume v, and temperature t. The ideal gas law was first written in 1834 by emil clapeyron. This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. To derive the ideal gas equation, we combine the three basic gas laws: For a static sample of gas, we can write each of the six gas laws as follows: The ideal gas equation can be written as. The ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii How To Derive Ideal Gas Law To derive the ideal gas equation, we combine the three basic gas laws: Suppose a gas consisting of n moles is at a pressure p, volume v, and temperature t. The ideal gas equation can be written as. (a) when air is pumped into a deflated tire, its volume first increases without much increase in pressure. The ideal gas law. How To Derive Ideal Gas Law.
From atomstalk.com
The Gas Laws AtomsTalk How To Derive Ideal Gas Law V is the volume of the ideal gas. From boyle’s law, p ∝ 1/v. To derive the ideal gas equation, we combine the three basic gas laws: Boyle’s law, charles’ law, and avogadro’s law. This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. The ideal gas law. How To Derive Ideal Gas Law.
From sciencenotes.org
Ideal Gas Law Formula and Examples How To Derive Ideal Gas Law This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. The ideal gas law was first written in 1834 by emil clapeyron. V is the volume of the ideal gas. It is a good approximation to the behaviour of many gases under many conditions, although it has several. How To Derive Ideal Gas Law.
From owlcation.com
The Theories and Behavior of Gas Owlcation How To Derive Ideal Gas Law This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. To derive the ideal gas equation, we combine the three basic gas laws: What follows is just one way to derive the ideal gas law. (a) when air is pumped into a deflated tire, its volume first increases. How To Derive Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii How To Derive Ideal Gas Law This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. The ideal gas law was first written in 1834 by emil clapeyron. To derive the ideal gas equation, we combine the three basic gas laws: Where, p is the pressure of the ideal gas. From boyle’s law, p. How To Derive Ideal Gas Law.
From www.youtube.com
General Chemistry Ideal Gas Law (PV=nRT) [Example 2] YouTube How To Derive Ideal Gas Law Suppose a gas consisting of n moles is at a pressure p, volume v, and temperature t. (a) when air is pumped into a deflated tire, its volume first increases without much increase in pressure. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. What follows is just one. How To Derive Ideal Gas Law.
From www.sliderbase.com
Gas Laws Presentation Chemistry How To Derive Ideal Gas Law From charles’ law, v ∝ t. The ideal gas law is the equation of state of a hypothetical ideal gas. (b) when the tire is. The ideal gas law was first written in 1834 by emil clapeyron. The ideal gas equation can be written as. (a) when air is pumped into a deflated tire, its volume first increases without much. How To Derive Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7067134 How To Derive Ideal Gas Law (b) when the tire is. The ideal gas law was first written in 1834 by emil clapeyron. The ideal gas equation can be written as. What follows is just one way to derive the ideal gas law. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. From boyle’s law,. How To Derive Ideal Gas Law.
From www.showme.com
Ideal gas law derivation Science, Pchem ShowMe How To Derive Ideal Gas Law From boyle’s law, p ∝ 1/v. V is the volume of the ideal gas. Suppose a gas consisting of n moles is at a pressure p, volume v, and temperature t. (a) when air is pumped into a deflated tire, its volume first increases without much increase in pressure. For a static sample of gas, we can write each of. How To Derive Ideal Gas Law.
From www.slideserve.com
PPT Characteristics of Gases PowerPoint Presentation, free download How To Derive Ideal Gas Law V is the volume of the ideal gas. From boyle’s law, p ∝ 1/v. This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. For a static sample of gas, we can write each of the six gas laws as follows: The ideal gas law is simply the. How To Derive Ideal Gas Law.
From mmerevise.co.uk
The Ideal Gas Equation MME How To Derive Ideal Gas Law For a static sample of gas, we can write each of the six gas laws as follows: The ideal gas law is the equation of state of a hypothetical ideal gas. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. V is the volume of the ideal gas. To. How To Derive Ideal Gas Law.
From studylib.net
Ideal Gas Law How To Derive Ideal Gas Law From boyle’s law, p ∝ 1/v. The ideal gas law is the equation of state of a hypothetical ideal gas. Boyle’s law, charles’ law, and avogadro’s law. This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. The ideal gas law describes the behavior of an ideal gas,. How To Derive Ideal Gas Law.
From www.youtube.com
Ideal Gas Equation (PV=nRT) Explanation and Examples YouTube How To Derive Ideal Gas Law The ideal gas law is the equation of state of a hypothetical ideal gas. The ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so. From charles’ law, v ∝ t. V is the volume of the ideal gas. This tutorial will teach you about the gas laws, the. How To Derive Ideal Gas Law.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net How To Derive Ideal Gas Law (b) when the tire is. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. For a static sample of gas, we can write each of the six gas laws as follows: The ideal gas law is the equation of state of a hypothetical ideal gas. Suppose a gas consisting. How To Derive Ideal Gas Law.
From www.youtube.com
Gas density and PV=nRT, the ideal gas law YouTube How To Derive Ideal Gas Law Suppose a gas consisting of n moles is at a pressure p, volume v, and temperature t. What follows is just one way to derive the ideal gas law. Where, p is the pressure of the ideal gas. From charles’ law, v ∝ t. The ideal gas law is simply the combination of all simple gas laws (boyle's law, charles'. How To Derive Ideal Gas Law.
From chemistryskills.com
Ideal Gas Law Combined Gas Law Chemistry Skills How To Derive Ideal Gas Law The ideal gas law is the equation of state of a hypothetical ideal gas. The ideal gas equation can be written as. It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. To derive the ideal gas equation, we combine the three basic gas laws: Where, p is the pressure of. How To Derive Ideal Gas Law.
From brainly.in
derive the ideal gas equation. Brainly.in How To Derive Ideal Gas Law What follows is just one way to derive the ideal gas law. From boyle’s law, p ∝ 1/v. The ideal gas law was first written in 1834 by emil clapeyron. (b) when the tire is. This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. For a static. How To Derive Ideal Gas Law.
From www.youtube.com
Deriving the combined and Ideal gas Laws (part 2) YouTube How To Derive Ideal Gas Law Boyle’s law, charles’ law, and avogadro’s law. This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. The ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so. Where, p is the pressure of the ideal gas.. How To Derive Ideal Gas Law.
From mydigitalkemistry.com
Ideal gas law derivation Combined Gas Laws Gases Best Online Free How To Derive Ideal Gas Law V is the volume of the ideal gas. The ideal gas law was first written in 1834 by emil clapeyron. The ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so. The ideal gas law is the equation of state of a hypothetical ideal gas. For a static sample. How To Derive Ideal Gas Law.
From testbook.com
Derivation of Ideal Gas Equation Boyle's, Charle's, Avogadro Law How To Derive Ideal Gas Law This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. To derive the ideal gas equation, we combine the three basic gas laws: The ideal gas law was. How To Derive Ideal Gas Law.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica How To Derive Ideal Gas Law The ideal gas law was first written in 1834 by emil clapeyron. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. Suppose a gas consisting of n moles is at a pressure p, volume v, and temperature t. (a) when air is pumped into a deflated tire, its volume. How To Derive Ideal Gas Law.
From mavink.com
Derive Ideal Gas Equation How To Derive Ideal Gas Law The ideal gas law was first written in 1834 by emil clapeyron. (a) when air is pumped into a deflated tire, its volume first increases without much increase in pressure. To derive the ideal gas equation, we combine the three basic gas laws: What follows is just one way to derive the ideal gas law. The ideal gas law is. How To Derive Ideal Gas Law.
From www.vecteezy.com
Ideal Gas Law formula. Boyle law, charles law, combined law vector How To Derive Ideal Gas Law (b) when the tire is. It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. Suppose a gas consisting of n moles is at a pressure p, volume v, and temperature t. V is the volume of the ideal gas. This tutorial will teach you about the gas laws, the derivation. How To Derive Ideal Gas Law.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation How To Derive Ideal Gas Law The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. (b) when the tire is. From boyle’s law, p ∝ 1/v. The ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so. Where, p is the pressure of the. How To Derive Ideal Gas Law.
From www.youtube.com
Ideal Gas Equation Crash Course Chemistry 12 Derivation of pv=nrt How To Derive Ideal Gas Law This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. Where, p is the pressure of the ideal gas. The ideal gas law was first written in 1834 by emil clapeyron. Boyle’s law, charles’ law, and avogadro’s law. The ideal gas law is the equation of state of. How To Derive Ideal Gas Law.
From www.pinterest.com
Combined Gas Law Definition, Formula, Examples Boyle's law, Ideal How To Derive Ideal Gas Law Suppose a gas consisting of n moles is at a pressure p, volume v, and temperature t. (b) when the tire is. For a static sample of gas, we can write each of the six gas laws as follows: To derive the ideal gas equation, we combine the three basic gas laws: Where, p is the pressure of the ideal. How To Derive Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 How To Derive Ideal Gas Law Where, p is the pressure of the ideal gas. Boyle’s law, charles’ law, and avogadro’s law. To derive the ideal gas equation, we combine the three basic gas laws: This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. From boyle’s law, p ∝ 1/v. The ideal gas. How To Derive Ideal Gas Law.
From www.youtube.com
DERIVATION OF IDEAL GAS EQUATION PV=mRT PV=nRT IDEAL GAS LAW How To Derive Ideal Gas Law The ideal gas law was first written in 1834 by emil clapeyron. The ideal gas equation can be written as. The ideal gas law is the equation of state of a hypothetical ideal gas. This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use it. (a) when air is. How To Derive Ideal Gas Law.
From www.youtube.com
Ideal Gas Equation Derivation and its different forms YouTube How To Derive Ideal Gas Law The ideal gas law was first written in 1834 by emil clapeyron. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. Boyle’s law, charles’ law, and avogadro’s law. This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and how to use. How To Derive Ideal Gas Law.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 How To Derive Ideal Gas Law The ideal gas law was first written in 1834 by emil clapeyron. From charles’ law, v ∝ t. What follows is just one way to derive the ideal gas law. To derive the ideal gas equation, we combine the three basic gas laws: V is the volume of the ideal gas. The ideal gas law is simply the combination of. How To Derive Ideal Gas Law.
From general.chemistrysteps.com
The Ideal Gas Law Chemistry Steps How To Derive Ideal Gas Law From boyle’s law, p ∝ 1/v. From charles’ law, v ∝ t. Boyle’s law, charles’ law, and avogadro’s law. To derive the ideal gas equation, we combine the three basic gas laws: The ideal gas law is the equation of state of a hypothetical ideal gas. V is the volume of the ideal gas. What follows is just one way. How To Derive Ideal Gas Law.
From chemistnotes.com
Ideal Gas Law (PV = nRT) Derivation and Numerical Values of R How To Derive Ideal Gas Law For a static sample of gas, we can write each of the six gas laws as follows: V is the volume of the ideal gas. The ideal gas law is the equation of state of a hypothetical ideal gas. To derive the ideal gas equation, we combine the three basic gas laws: Boyle’s law, charles’ law, and avogadro’s law. The. How To Derive Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID6624513 How To Derive Ideal Gas Law For a static sample of gas, we can write each of the six gas laws as follows: It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. From charles’ law, v ∝ t. This tutorial will teach you about the gas laws, the derivation of the ideal gas law equation, and. How To Derive Ideal Gas Law.
From learncheme.com
idealgaslawconceptest1 LearnChemE How To Derive Ideal Gas Law The ideal gas law is simply the combination of all simple gas laws (boyle's law, charles' law, and avogadro's law), and so. What follows is just one way to derive the ideal gas law. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively. Suppose a gas consisting of n. How To Derive Ideal Gas Law.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID How To Derive Ideal Gas Law It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. The ideal gas equation can be written as. Boyle’s law, charles’ law, and avogadro’s law. From charles’ law, v ∝ t. (a) when air is pumped into a deflated tire, its volume first increases without much increase in pressure. (b) when. How To Derive Ideal Gas Law.