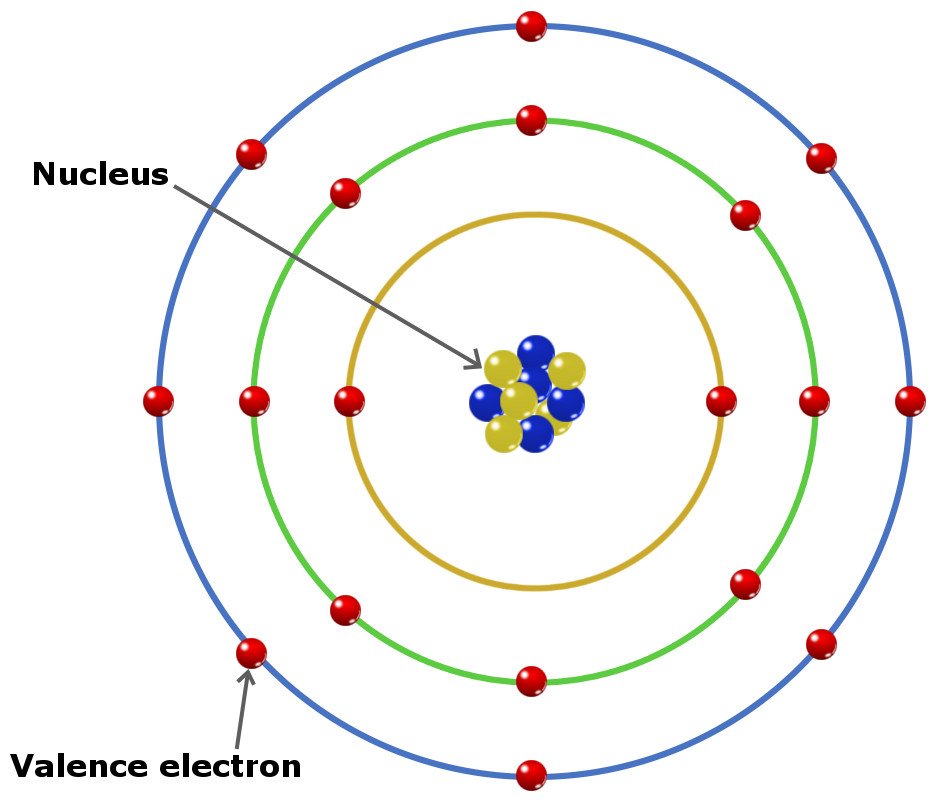
What Is Unique About The Valence Electron Shell Configuration . A full valence shell is the most stable. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. The valence electrons, electrons in the outermost shell, are the. They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. That is why elements whose atoms have the same number. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. As they are farthest from the nucleus, they are loosely attached to it. Before assigning the electrons of an atom into orbitals, one must. The valence electrons are the electrons in the outermost electron shell of an atom. Valence electrons are outer shell electrons for main group elements. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell.
from www.expii.com
A full valence shell is the most stable. Valence electrons are outer shell electrons for main group elements. The valence electrons are the electrons in the outermost electron shell of an atom. Before assigning the electrons of an atom into orbitals, one must. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). As they are farthest from the nucleus, they are loosely attached to it.
Valence Electrons — Definition & Importance Expii
What Is Unique About The Valence Electron Shell Configuration As they are farthest from the nucleus, they are loosely attached to it. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The valence electrons are the electrons in the outermost electron shell of an atom. As they are farthest from the nucleus, they are loosely attached to it. They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. Before assigning the electrons of an atom into orbitals, one must. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Valence electrons are outer shell electrons for main group elements. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. That is why elements whose atoms have the same number. A full valence shell is the most stable. The valence electrons, electrons in the outermost shell, are the. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell).
From revision.my
2.4.1 Electron Arrangement Revision.my What Is Unique About The Valence Electron Shell Configuration The valence electrons, electrons in the outermost shell, are the. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Valence electrons are outer shell electrons for main group elements. The valence electrons are the electrons in the outermost electron shell of an atom. Before assigning the electrons of an atom. What Is Unique About The Valence Electron Shell Configuration.
From biologydictionary.net
Covalent Bond Biology Dictionary What Is Unique About The Valence Electron Shell Configuration The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Valence electrons are outer shell electrons for main group elements. Before assigning the electrons of an atom into orbitals, one must. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. As. What Is Unique About The Valence Electron Shell Configuration.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell What Is Unique About The Valence Electron Shell Configuration Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. The valence electrons are the electrons in the outermost electron shell of an atom. Valence electrons are located in an atomic nucleus ’s outermost shell. What Is Unique About The Valence Electron Shell Configuration.
From www.allaboutcircuits.com
Valence and Crystal Structure Solidstate Device Theory Electronics What Is Unique About The Valence Electron Shell Configuration Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The valence electrons are the electrons in the outermost electron shell of an atom. Before assigning the electrons of an atom into orbitals, one. What Is Unique About The Valence Electron Shell Configuration.
From www.goodscience.com.au
Atoms, Elements and Compounds Good Science What Is Unique About The Valence Electron Shell Configuration They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Valence electrons are outer shell electrons for main group elements. Many of the physical and chemical properties of elements can be correlated to. What Is Unique About The Valence Electron Shell Configuration.
From gzscienceclassonline.weebly.com
1. Electron Configuration What Is Unique About The Valence Electron Shell Configuration The valence electrons, electrons in the outermost shell, are the. Before assigning the electrons of an atom into orbitals, one must. Valence electrons are outer shell electrons for main group elements. A full valence shell is the most stable. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons are. What Is Unique About The Valence Electron Shell Configuration.
From www.youtube.com
Valence Electrons & Electron Configurations YouTube What Is Unique About The Valence Electron Shell Configuration The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. A full valence shell is the most stable. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). The valence electrons, electrons in the outermost shell, are the. Group 18 elements (helium, neon, and argon are shown. What Is Unique About The Valence Electron Shell Configuration.
From chemistry291.blogspot.com
[5 Steps] Electronic Configuration of Fluorine(F) What Is Unique About The Valence Electron Shell Configuration Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. A full valence shell is the most stable. That is why elements whose atoms have the same number. Before assigning the electrons of an atom into orbitals, one must. The valence electrons are the electrons in the outermost electron shell of an atom.. What Is Unique About The Valence Electron Shell Configuration.
From mavink.com
Valence Electron Orbital Diagram What Is Unique About The Valence Electron Shell Configuration That is why elements whose atoms have the same number. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. Valence electrons are outer shell electrons for main group elements. The valence electrons,. What Is Unique About The Valence Electron Shell Configuration.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is Unique About The Valence Electron Shell Configuration The valence electrons, electrons in the outermost shell, are the. That is why elements whose atoms have the same number. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. As they are farthest from the nucleus, they are loosely attached to it. Before assigning the electrons of an atom into. What Is Unique About The Valence Electron Shell Configuration.
From byjus.com
Different energy shells, valence shell, valence electrons What Is Unique About The Valence Electron Shell Configuration Before assigning the electrons of an atom into orbitals, one must. As they are farthest from the nucleus, they are loosely attached to it. They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the. What Is Unique About The Valence Electron Shell Configuration.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes What Is Unique About The Valence Electron Shell Configuration Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. That is why elements whose atoms have the same number. As they are farthest from the nucleus, they are loosely attached to it. Valence electrons. What Is Unique About The Valence Electron Shell Configuration.
From www.youtube.com
Valence Electrons and the Periodic Table YouTube What Is Unique About The Valence Electron Shell Configuration The valence electrons are the electrons in the outermost electron shell of an atom. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. Valence electrons are outer shell electrons for main group elements. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). As they are farthest from. What Is Unique About The Valence Electron Shell Configuration.
From praxilabs.com
Your Step by Step Guide to Find Valence Electrons praxilabs What Is Unique About The Valence Electron Shell Configuration A full valence shell is the most stable. Valence electrons are outer shell electrons for main group elements. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). Before assigning the electrons of an atom into orbitals,. What Is Unique About The Valence Electron Shell Configuration.
From www.youtube.com
Number of Valence Electrons for Hydrogen (H) YouTube What Is Unique About The Valence Electron Shell Configuration That is why elements whose atoms have the same number. The valence electrons, electrons in the outermost shell, are the. A full valence shell is the most stable. As they are farthest from the nucleus, they are loosely attached to it. They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. Valence. What Is Unique About The Valence Electron Shell Configuration.
From sciencenotes.org
List of Electron Configurations of Elements What Is Unique About The Valence Electron Shell Configuration A full valence shell is the most stable. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. They actively participate in all its. What Is Unique About The Valence Electron Shell Configuration.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Is Unique About The Valence Electron Shell Configuration The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). A full valence shell is the most stable. The valence electrons, electrons in the outermost shell, are the. Before assigning the electrons of an atom into orbitals,. What Is Unique About The Valence Electron Shell Configuration.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Is Unique About The Valence Electron Shell Configuration They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). As they are farthest from the nucleus, they are loosely attached to it. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. What Is Unique About The Valence Electron Shell Configuration.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is Unique About The Valence Electron Shell Configuration They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. As they are farthest from the nucleus, they are loosely attached to it. The valence electrons are the electrons in the outermost electron shell of an atom. Valence electrons are outer shell electrons for main group elements. Many of the physical and. What Is Unique About The Valence Electron Shell Configuration.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary What Is Unique About The Valence Electron Shell Configuration As they are farthest from the nucleus, they are loosely attached to it. Before assigning the electrons of an atom into orbitals, one must. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). The valence electrons are the electrons in the outermost electron shell of an atom. Valence electrons are outer shell electrons for main. What Is Unique About The Valence Electron Shell Configuration.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is Unique About The Valence Electron Shell Configuration Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. A full valence shell is the most stable. Valence electrons are outer shell electrons for main group elements. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). That is why elements whose atoms have the same. What Is Unique About The Valence Electron Shell Configuration.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is Unique About The Valence Electron Shell Configuration Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Before assigning the electrons of an atom into orbitals, one must. Valence electrons are outer shell electrons for main group elements. The. What Is Unique About The Valence Electron Shell Configuration.
From www.animalia-life.club
Electron Shell Diagram What Is Unique About The Valence Electron Shell Configuration Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. Before assigning the electrons of an atom into orbitals, one must. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). A full valence shell is the most stable. Valence electrons are outer shell electrons for main group elements.. What Is Unique About The Valence Electron Shell Configuration.
From mungfali.com
Valence Electron Shell Diagram What Is Unique About The Valence Electron Shell Configuration They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. A full valence shell is the most stable. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). That. What Is Unique About The Valence Electron Shell Configuration.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry What Is Unique About The Valence Electron Shell Configuration Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. Valence electrons are outer shell electrons for main group elements. They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. The valence electrons are the electrons in the outermost electron shell of an atom. The. What Is Unique About The Valence Electron Shell Configuration.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free What Is Unique About The Valence Electron Shell Configuration As they are farthest from the nucleus, they are loosely attached to it. A full valence shell is the most stable. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. The valence electrons are the electrons in the outermost electron shell of an atom. Many of the physical and chemical. What Is Unique About The Valence Electron Shell Configuration.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is Unique About The Valence Electron Shell Configuration The valence electrons, electrons in the outermost shell, are the. A full valence shell is the most stable. That is why elements whose atoms have the same number. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). Valence electrons are outer shell electrons for main group elements. The valence electrons, electrons in the outermost shell,. What Is Unique About The Valence Electron Shell Configuration.
From www.focuskimia.com
General Chemistry Review Valence Electrons of The First Row Elements What Is Unique About The Valence Electron Shell Configuration That is why elements whose atoms have the same number. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Valence electrons are outer shell electrons for main group elements. Valence electrons. What Is Unique About The Valence Electron Shell Configuration.
From mavink.com
Periodic Table Valence Electrons What Is Unique About The Valence Electron Shell Configuration The valence electrons, electrons in the outermost shell, are the. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. Valence electrons are outer shell electrons for main group elements. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. They actively participate in. What Is Unique About The Valence Electron Shell Configuration.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is Unique About The Valence Electron Shell Configuration The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Valence electrons are outer shell electrons for main group elements. The valence electrons, electrons in the outermost shell, are the. That is why elements whose atoms have the same number. A full valence shell is the most stable. They actively participate. What Is Unique About The Valence Electron Shell Configuration.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Is Unique About The Valence Electron Shell Configuration A full valence shell is the most stable. Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). Valence electrons are outer shell electrons for main group elements. As they are farthest from the nucleus, they are loosely attached to it. That is why elements whose atoms have the same number. Before assigning the electrons of. What Is Unique About The Valence Electron Shell Configuration.
From surfguppy.com
Valence Electrons Definition, Obits and Energy Level What Is Unique About The Valence Electron Shell Configuration Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. That is why elements whose atoms have the same number. As they are farthest from the nucleus, they are loosely attached to it. Valence electrons are outer shell electrons for main group elements. Valence electrons are located in an atomic nucleus ’s outermost. What Is Unique About The Valence Electron Shell Configuration.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Is Unique About The Valence Electron Shell Configuration As they are farthest from the nucleus, they are loosely attached to it. The valence electrons are the electrons in the outermost electron shell of an atom. Valence electrons are outer shell electrons for main group elements. That is why elements whose atoms have the same number. Many of the physical and chemical properties of elements can be correlated to. What Is Unique About The Valence Electron Shell Configuration.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is Unique About The Valence Electron Shell Configuration A full valence shell is the most stable. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Valence electrons are outer shell electrons for main group elements. They actively participate in all its reactions by forming bonds with the valence electron of the adjacent atoms. The valence electrons are the. What Is Unique About The Valence Electron Shell Configuration.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science What Is Unique About The Valence Electron Shell Configuration Valence electrons are outer shell electrons for main group elements. The valence electrons, electrons in the outermost shell, are the. That is why elements whose atoms have the same number. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the determining factor for. What Is Unique About The Valence Electron Shell Configuration.