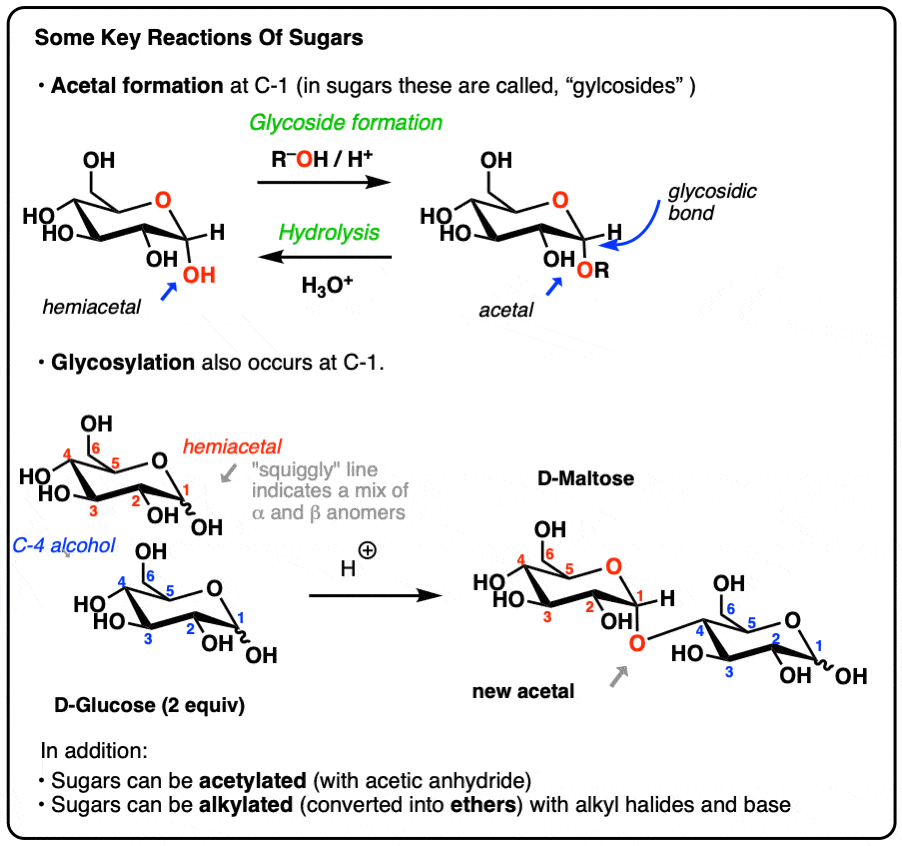
Sucrose Hydrolysis Mechanism . The mechanism really isn't much more complicated than what you describe. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. The $\ce{h+}$ binds to the acetal linkage. A heterolysis step may yield a. A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. Water then binds to the fructose ring,. Most of these models suggest a michaelis. A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of.
from proper-cooking.info
The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. The $\ce{h+}$ binds to the acetal linkage. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. A heterolysis step may yield a. Most of these models suggest a michaelis. Water then binds to the fructose ring,. The mechanism really isn't much more complicated than what you describe.
Sucrose Hydrolysis Mechanism
Sucrose Hydrolysis Mechanism The $\ce{h+}$ binds to the acetal linkage. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. Most of these models suggest a michaelis. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. The $\ce{h+}$ binds to the acetal linkage. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. Water then binds to the fructose ring,. A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. A heterolysis step may yield a. The mechanism really isn't much more complicated than what you describe. A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and.
From www.researchgate.net
Isomerization and hydrolysis of sucrose as catalyzed by sucrose Sucrose Hydrolysis Mechanism A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. The mechanism really isn't much more complicated than what you describe. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. Various kinetic models have been proposed to describe the hydrolysis of sucrose. Sucrose Hydrolysis Mechanism.
From www.alamy.com
Sucrase enzyme Effect On Sucrose Sugar Molecule. Sucrose Hydrolysis Sucrose Hydrolysis Mechanism Water then binds to the fructose ring,. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. A. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. Water then binds to the fructose ring,. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. The mechanism really isn't much more complicated than what you describe. A heterolysis step may yield a.. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. Most of these models suggest a michaelis. The $\ce{h+}$ binds to the acetal linkage. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism. Sucrose Hydrolysis Mechanism.
From www.researchgate.net
Proposed mechanism for the hydrolysis of phosphorylated isomers of Sucrose Hydrolysis Mechanism Most of these models suggest a michaelis. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. Water then binds to the fructose ring,. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. A heterolysis step may. Sucrose Hydrolysis Mechanism.
From emmaleeewasolomon.blogspot.com
Hydrolysis of Sucrose EmmaleeewaSolomon Sucrose Hydrolysis Mechanism The $\ce{h+}$ binds to the acetal linkage. A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. The hydrolysis of sucrose. Sucrose Hydrolysis Mechanism.
From www.slideserve.com
PPT Organic Molecules The Building Blocks of Life PowerPoint Sucrose Hydrolysis Mechanism Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. Water then binds to the fructose ring,. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. A. Sucrose Hydrolysis Mechanism.
From tukioka-clinic.com
🎉 Hydrolysis of sucrose. sucrose hydolysis by sucrase. 20190127 Sucrose Hydrolysis Mechanism The $\ce{h+}$ binds to the acetal linkage. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. Water then binds to the fructose ring,. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. It may be concluded. Sucrose Hydrolysis Mechanism.
From sydizarmo.weebly.com
Acidhydrolysisofsucrose VERIFIED Sucrose Hydrolysis Mechanism The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. The mechanism really isn't much more complicated than what you describe. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. A reasonable mechanism starts with a proton transfer step at the. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. A heterolysis step may yield a. The $\ce{h+}$ binds to the acetal linkage. The hydrolysis of sucrose in dilute acid or through the action. Sucrose Hydrolysis Mechanism.
From chemistry.stackovernet.com
organicchemistry ¿Por qué se utiliza HCl en la hidrólisis de la Sucrose Hydrolysis Mechanism Water then binds to the fructose ring,. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. Most of these models suggest a michaelis. A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. The mechanism really isn't much more complicated than what you. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. The $\ce{h+}$ binds to the acetal linkage. Most of these models suggest a michaelis. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. It may be concluded that the hydrolysis of sucrose catalyzed by. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism Most of these models suggest a michaelis. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. The $\ce{h+}$ binds to the acetal linkage. A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism Water then binds to the fructose ring,. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the. Sucrose Hydrolysis Mechanism.
From www.pdfprof.com
acid hydrolysis of sucrose Sucrose Hydrolysis Mechanism Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. A heterolysis step may yield a. Water then binds to the fructose ring,. Measurements were performed during both isothermal conditions and during slow heating and then cooling at. Sucrose Hydrolysis Mechanism.
From www.pdfprof.com
acid catalyzed hydrolysis of sucrose Sucrose Hydrolysis Mechanism Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. A heterolysis step may yield a. Most of these models suggest a michaelis. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. The mechanism really isn't much more complicated than what. Sucrose Hydrolysis Mechanism.
From slideserve.com
PPT Cyclic Structure of Fructose PowerPoint Presentation ID342112 Sucrose Hydrolysis Mechanism The mechanism really isn't much more complicated than what you describe. A heterolysis step may yield a. Water then binds to the fructose ring,. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. A thermodynamic investigation of. Sucrose Hydrolysis Mechanism.
From www.pinterest.com
Chemicals and Macromolecules Anatomy and Physiology (OLI import Sucrose Hydrolysis Mechanism Water then binds to the fructose ring,. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. A heterolysis step may yield a. A reasonable mechanism starts with. Sucrose Hydrolysis Mechanism.
From glossary.periodni.com
Sucrose Chemistry Dictionary & Glossary Sucrose Hydrolysis Mechanism A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. Water then binds to the fructose ring,. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. The $\ce{h+}$ binds to the acetal linkage. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. Most. Sucrose Hydrolysis Mechanism.
From bio1151.nicerweb.com
sucrase.html 08_13HydrolysisOfSucrose.jpg Sucrose Hydrolysis Mechanism A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. The $\ce{h+}$ binds to the acetal linkage. Water then binds to the fructose ring,. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. A reasonable mechanism starts. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. Water then binds to the fructose ring,. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. Measurements were. Sucrose Hydrolysis Mechanism.
From www.chegg.com
Solved For the mechanism of the hydrolysis of sucrose to Sucrose Hydrolysis Mechanism A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. The $\ce{h+}$ binds to the acetal linkage. A heterolysis step may yield a. Most of these models suggest a michaelis. The mechanism really isn't much more complicated than what you describe. Measurements were performed during both isothermal conditions and during slow heating and. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism The $\ce{h+}$ binds to the acetal linkage. The mechanism really isn't much more complicated than what you describe. Water then binds to the fructose ring,. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the. Sucrose Hydrolysis Mechanism.
From www.researchgate.net
Sucrose hydrolysis reaction. Download Scientific Diagram Sucrose Hydrolysis Mechanism A reasonable mechanism starts with a proton transfer step at the oxgen atom joining the two rings. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. The $\ce{h+}$ binds to the acetal linkage. Most of these models suggest a michaelis. Measurements were performed during both isothermal conditions and during. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. Most of these models suggest a michaelis. A heterolysis step may yield a. The $\ce{h+}$ binds to the acetal linkage. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. A thermodynamic. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism Most of these models suggest a michaelis. A heterolysis step may yield a. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. The $\ce{h+}$ binds to the acetal linkage. The mechanism really isn't much more complicated than what you describe. Water then binds to the fructose ring,. It may be concluded. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism Water then binds to the fructose ring,. The mechanism really isn't much more complicated than what you describe. A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. The $\ce{h+}$ binds to the acetal linkage. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. The hydrolysis of. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. Water then binds to the fructose ring,. Most of these models suggest a michaelis. The $\ce{h+}$ binds to the acetal linkage. It. Sucrose Hydrolysis Mechanism.
From www.researchgate.net
Schematic representation of sucrose and trehalose hydrolysis. Sucrose Sucrose Hydrolysis Mechanism The $\ce{h+}$ binds to the acetal linkage. Water then binds to the fructose ring,. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. The hydrolysis of sucrose in dilute acid or. Sucrose Hydrolysis Mechanism.
From proper-cooking.info
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism A heterolysis step may yield a. Most of these models suggest a michaelis. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. Water then binds to the fructose ring,. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. A thermodynamic investigation of the hydrolysis of sucrose. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a controlled rate. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and. The mechanism really isn't much more complicated than what you describe. It may be concluded that the hydrolysis of sucrose catalyzed by invertase has a mechanism comparable to that of acid. The $\ce{h+}$ binds to the acetal linkage. The hydrolysis of sucrose. Sucrose Hydrolysis Mechanism.
From ar.inspiredpencil.com
Sucrose Hydrolysis Mechanism Sucrose Hydrolysis Mechanism The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. The mechanism really isn't much more complicated than what you describe. Various kinetic models have been proposed to describe the hydrolysis of sucrose by invertase. A heterolysis step may yield a. Most of these models suggest. Sucrose Hydrolysis Mechanism.
From www.pdfprof.com
acid catalyzed hydrolysis of sucrose mechanism Sucrose Hydrolysis Mechanism A heterolysis step may yield a. The $\ce{h+}$ binds to the acetal linkage. The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of. Water then binds to the fructose ring,. Measurements were performed during both isothermal conditions and during slow heating and then cooling at a. Sucrose Hydrolysis Mechanism.