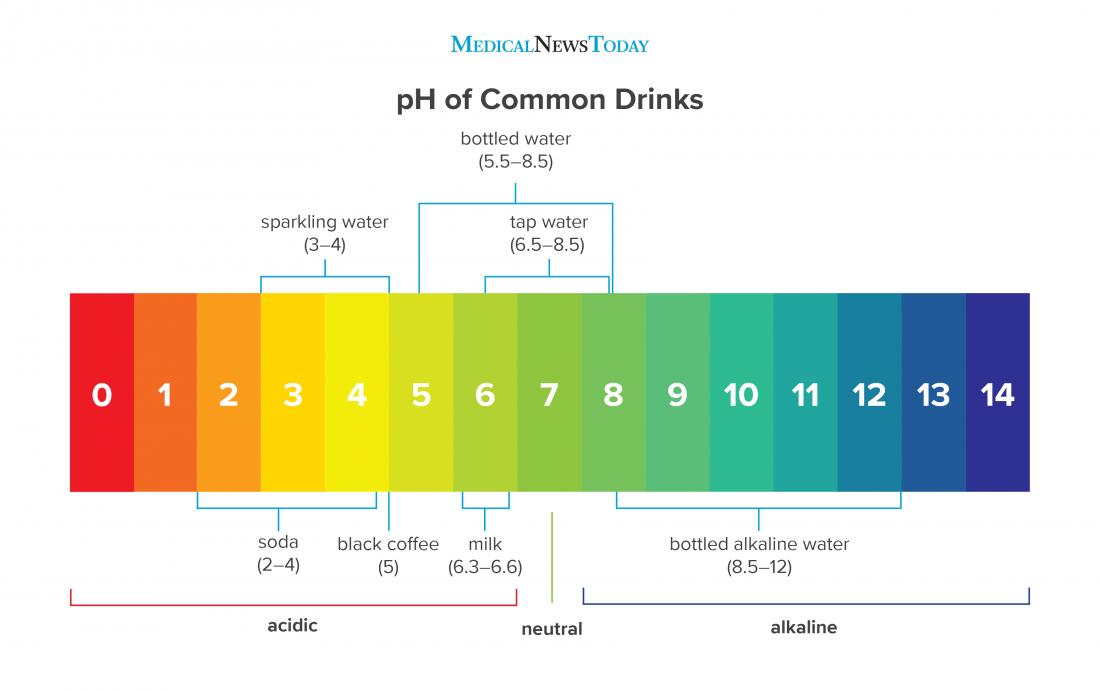
Does Lemon Lower Ph . The ph level of a substance is measured on a scale from 0 to 14. This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and slightly more. The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill bacteria. When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. Lemon juice ph is around 2.0, ranging between 2 and 3. Using lemon juice to lower the ph in water provides a natural and effective alternative to harsh chemicals. Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. The ph level of lemon juice can vary slightly depending. Pure water has a ph of 7 and is considered neutral. According to north dakota state university, lemon juice is often used in. Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. The high acidity is responsible for the tangy taste and sour flavor of lemons. Lemons have an acidic ph because they contain a high amount of citric acid.
from www.medicalnewstoday.com
Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. The high acidity is responsible for the tangy taste and sour flavor of lemons. Using lemon juice to lower the ph in water provides a natural and effective alternative to harsh chemicals. When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. Lemon juice ph is around 2.0, ranging between 2 and 3. Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. The ph level of lemon juice can vary slightly depending. Pure water has a ph of 7 and is considered neutral. The ph level of a substance is measured on a scale from 0 to 14. Lemons have an acidic ph because they contain a high amount of citric acid.
What to know about the pH of water
Does Lemon Lower Ph Pure water has a ph of 7 and is considered neutral. The high acidity is responsible for the tangy taste and sour flavor of lemons. Lemons have an acidic ph because they contain a high amount of citric acid. Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. Using lemon juice to lower the ph in water provides a natural and effective alternative to harsh chemicals. Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill bacteria. Lemon juice ph is around 2.0, ranging between 2 and 3. The ph level of a substance is measured on a scale from 0 to 14. According to north dakota state university, lemon juice is often used in. The ph level of lemon juice can vary slightly depending. Pure water has a ph of 7 and is considered neutral. This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and slightly more.
From brainly.com
I WILL GIVE BRAINLIEST!!! The scale below shows the pH levels of Does Lemon Lower Ph This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and slightly more. Lemon juice ph is around 2.0, ranging between 2 and 3. The ph level of lemon juice can vary slightly depending. Lemons have an acidic ph because they contain a high amount of citric acid. Using lemon juice to lower the ph in. Does Lemon Lower Ph.
From juiceandjuicer.com
Why Does Lemon Juice Prevent Browning? An Explanation! Juice & Juicer Does Lemon Lower Ph When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. Lemons have an acidic ph because they contain a high amount of citric acid. The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill. Does Lemon Lower Ph.
From www.sciencelearn.org.nz
pH scale — Science Learning Hub Does Lemon Lower Ph The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill bacteria. Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. Using lemon juice to lower the ph in water provides a natural and effective alternative to harsh chemicals. Lemon juice is. Does Lemon Lower Ph.
From www.pinterest.com
Get benefits of lemon water by using lemonfuser water bottle Lemon Does Lemon Lower Ph This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and slightly more. Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. Lemon juice ph is around 2.0, ranging. Does Lemon Lower Ph.
From www.curlcentric.com
What Is the pH of Hair? Using pHBalanced Hair Products Does Lemon Lower Ph Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. Lemon juice ph is around 2.0, ranging between 2 and 3. Lemons have an acidic ph because they contain a high amount of citric acid. The ph level of a substance is measured on a scale from 0 to 14.. Does Lemon Lower Ph.
From www.mometrix.com
pH Overview (Chemistry Review Video) Does Lemon Lower Ph Using lemon juice to lower the ph in water provides a natural and effective alternative to harsh chemicals. Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and slightly more. Read on to discover the. Does Lemon Lower Ph.
From sciencenotes.org
The pH Scale of Common Chemicals Does Lemon Lower Ph When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. The high acidity is responsible for the tangy taste and sour flavor of lemons. Lemons have an acidic ph because they contain a high amount of citric acid. The ph level of a substance is measured. Does Lemon Lower Ph.
From techiescientist.com
pH of Lemon Acidic or Basic? Techiescientist Does Lemon Lower Ph When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. According to north dakota state university, lemon juice is often used in. The ph level of lemon juice can vary slightly depending. Lemon juice ph is around 2.0, ranging between 2 and 3. Lemons have an. Does Lemon Lower Ph.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG Does Lemon Lower Ph Pure water has a ph of 7 and is considered neutral. Lemon juice ph is around 2.0, ranging between 2 and 3. The ph level of lemon juice can vary slightly depending. The ph level of a substance is measured on a scale from 0 to 14. Read on to discover the ph of lemon juice and if it's considered. Does Lemon Lower Ph.
From blog.juicegrape.com
how to test for pH Archives Musto Wine Grape Company, LLC Does Lemon Lower Ph The ph level of lemon juice can vary slightly depending. According to north dakota state university, lemon juice is often used in. When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. The high acidity is responsible for the tangy taste and sour flavor of lemons.. Does Lemon Lower Ph.
From organicfacts.net
17 Best Benefits of Lemon Water Organic Facts Does Lemon Lower Ph Lemons have an acidic ph because they contain a high amount of citric acid. According to north dakota state university, lemon juice is often used in. Using lemon juice to lower the ph in water provides a natural and effective alternative to harsh chemicals. The ph level of lemon juice can vary slightly depending. The low ph of lemon juice. Does Lemon Lower Ph.
From www.emedihealth.com
Does Lemon Water Lower Cholesterol? eMediHealth Does Lemon Lower Ph Using lemon juice to lower the ph in water provides a natural and effective alternative to harsh chemicals. The ph level of a substance is measured on a scale from 0 to 14. Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. Lemon juice ph is around 2.0, ranging. Does Lemon Lower Ph.
From greenupside.com
Does Lemon Juice Lower pH? (How it Can Hurt Plants) GreenUpSide Does Lemon Lower Ph The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill bacteria. Lemon juice ph is around 2.0, ranging between 2 and 3. According to north dakota state university, lemon juice is often used in. The ph level of lemon juice can vary slightly depending. The high acidity is. Does Lemon Lower Ph.
From solherbsrecipe.blogspot.com
Lemon Water Ph Herbs and Food Recipes Does Lemon Lower Ph Using lemon juice to lower the ph in water provides a natural and effective alternative to harsh chemicals. Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. Lemons have an acidic ph because they contain a high amount of citric acid. The high acidity is responsible for the tangy taste and sour flavor. Does Lemon Lower Ph.
From solherbsrecipe.blogspot.com
Lemon Water Ph Herbs and Food Recipes Does Lemon Lower Ph The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill bacteria. Pure water has a ph of 7 and is considered neutral. The high acidity is responsible for the tangy taste and sour flavor of lemons. The ph level of a substance is measured on a scale from. Does Lemon Lower Ph.
From content.byui.edu
Acids, Bases, pH and Buffers Does Lemon Lower Ph Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. Lemon juice ph is around 2.0, ranging between 2 and 3. When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. This makes lemons less acidic (or more basic) than. Does Lemon Lower Ph.
From www.swimuniversity.com
How to Lower pH in a Pool Quickly with These Common Chemicals Does Lemon Lower Ph Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. The ph level of a substance is measured on a scale from 0 to 14. Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. This makes lemons less acidic (or more basic) than. Does Lemon Lower Ph.
From www.waterev.com
How to Lower pH In water Does Lemon Lower Ph The ph level of a substance is measured on a scale from 0 to 14. The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill bacteria. According to north dakota state university, lemon juice is often used in. Using lemon juice to lower the ph in water provides. Does Lemon Lower Ph.
From alaiynab.blogspot.com
Alaiyna B. Bath and Body pH testing of liquid soap and lowering pH Does Lemon Lower Ph When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. Using lemon juice to lower the ph in water provides a natural and effective alternative to harsh chemicals. Lemon juice ph is around 2.0, ranging between 2 and 3. Lemon juice is almost 100,000 times more. Does Lemon Lower Ph.
From www.pinterest.com
Initially, lemon juice is acidic. But when consumed, it metabolizes in Does Lemon Lower Ph Pure water has a ph of 7 and is considered neutral. According to north dakota state university, lemon juice is often used in. Using lemon juice to lower the ph in water provides a natural and effective alternative to harsh chemicals. The ph level of lemon juice can vary slightly depending. The low ph of lemon juice means that if. Does Lemon Lower Ph.
From justinboey.com
The Science Behind Face Moisturizers Should They Have a Low pH Does Lemon Lower Ph The ph level of a substance is measured on a scale from 0 to 14. This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and slightly more. Pure water has a ph of 7 and is considered neutral. Lemons have an acidic ph because they contain a high amount of citric acid. Read on to. Does Lemon Lower Ph.
From www.ucdavis.edu
Ocean Acidification UC Davis Does Lemon Lower Ph The ph level of lemon juice can vary slightly depending. Pure water has a ph of 7 and is considered neutral. Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. Lemon juice ph is around 2.0, ranging between 2 and 3. The high acidity is responsible for the tangy. Does Lemon Lower Ph.
From mavink.com
Ph Scale Acids And Bases Does Lemon Lower Ph Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. Lemon juice ph is around 2.0, ranging between 2 and 3. The ph level of lemon juice can vary slightly depending. The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low. Does Lemon Lower Ph.
From www.pinterest.com
Lemon water Lemon water, Health tips, Health benefits Does Lemon Lower Ph The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill bacteria. This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and slightly more. Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale.. Does Lemon Lower Ph.
From www.youtube.com
pH of Lemon Juice Is lemon juice acidic or alkaline? 🍋🍋🍋 YouTube Does Lemon Lower Ph Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. Pure water has a ph of 7 and is considered neutral. Lemons have an acidic ph because they contain a. Does Lemon Lower Ph.
From solherbsrecipe.blogspot.com
Lemon Water Ph Herbs and Food Recipes Does Lemon Lower Ph According to north dakota state university, lemon juice is often used in. This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and slightly more. Lemons have an acidic ph because they contain a high amount of citric acid. The high acidity is responsible for the tangy taste and sour flavor of lemons. Pure water has. Does Lemon Lower Ph.
From liveenergized.com
Alkaline Fruits Guide (Which Fruits Are Alkaline vs Acidic and Why) Does Lemon Lower Ph Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. The high acidity is responsible for the tangy taste and sour flavor of lemons. Pure water has a ph of 7 and is considered neutral. This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and. Does Lemon Lower Ph.
From www.medicalnewstoday.com
What to know about the pH of water Does Lemon Lower Ph The ph level of lemon juice can vary slightly depending. When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. Lemons have an acidic ph because they contain a high amount of citric acid. Read on to discover the ph of lemon juice and if it's. Does Lemon Lower Ph.
From solherbsrecipe.blogspot.com
Lemon Water Ph Herbs and Food Recipes Does Lemon Lower Ph Lemon juice is almost 100,000 times more acidic than water and falls between 2 and 3 on the ph scale. This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and slightly more. The ph level of lemon juice can vary slightly depending. When lemon juice is mixed with water, the resulting solution can have a. Does Lemon Lower Ph.
From juiceandjuicer.com
How Much Does A Lemon Weigh? Juice & Juicer Does Lemon Lower Ph The high acidity is responsible for the tangy taste and sour flavor of lemons. When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. This makes lemons less acidic (or. Does Lemon Lower Ph.
From preparatorychemistry.com
pH and Equilibrium Does Lemon Lower Ph The high acidity is responsible for the tangy taste and sour flavor of lemons. According to north dakota state university, lemon juice is often used in. When lemon juice is mixed with water, the resulting solution can have a ph level ranging from 2 to 3, making it highly acidic. Lemon juice is almost 100,000 times more acidic than water. Does Lemon Lower Ph.
From solherbsrecipe.blogspot.com
Lemon Water Ph Herbs and Food Recipes Does Lemon Lower Ph Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. Lemons have an acidic ph because they contain a high amount of citric acid. The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill bacteria. Pure water has a ph of 7. Does Lemon Lower Ph.
From www.inchcalculator.com
pH Calculator Inch Calculator Does Lemon Lower Ph The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill bacteria. Lemon juice ph is around 2.0, ranging between 2 and 3. Read on to discover the ph of lemon juice and if it's considered alkaline or acidic. According to north dakota state university, lemon juice is often. Does Lemon Lower Ph.
From byjus.com
pH Of Acids And Bases Calculate pH Value Chemistry Byju's Does Lemon Lower Ph Lemon juice ph is around 2.0, ranging between 2 and 3. The low ph of lemon juice means that if you add enough lemon juice to water, the acidity (low ph) will kill bacteria. The high acidity is responsible for the tangy taste and sour flavor of lemons. This makes lemons less acidic (or more basic) than battery acid (sulfuric. Does Lemon Lower Ph.
From thekitchenjournal.com
How Long Until Lemons Go Bad? Here's What You Should Know Does Lemon Lower Ph The ph level of a substance is measured on a scale from 0 to 14. This makes lemons less acidic (or more basic) than battery acid (sulfuric acid, ph=1.0) and slightly more. Lemon juice ph is around 2.0, ranging between 2 and 3. When lemon juice is mixed with water, the resulting solution can have a ph level ranging from. Does Lemon Lower Ph.