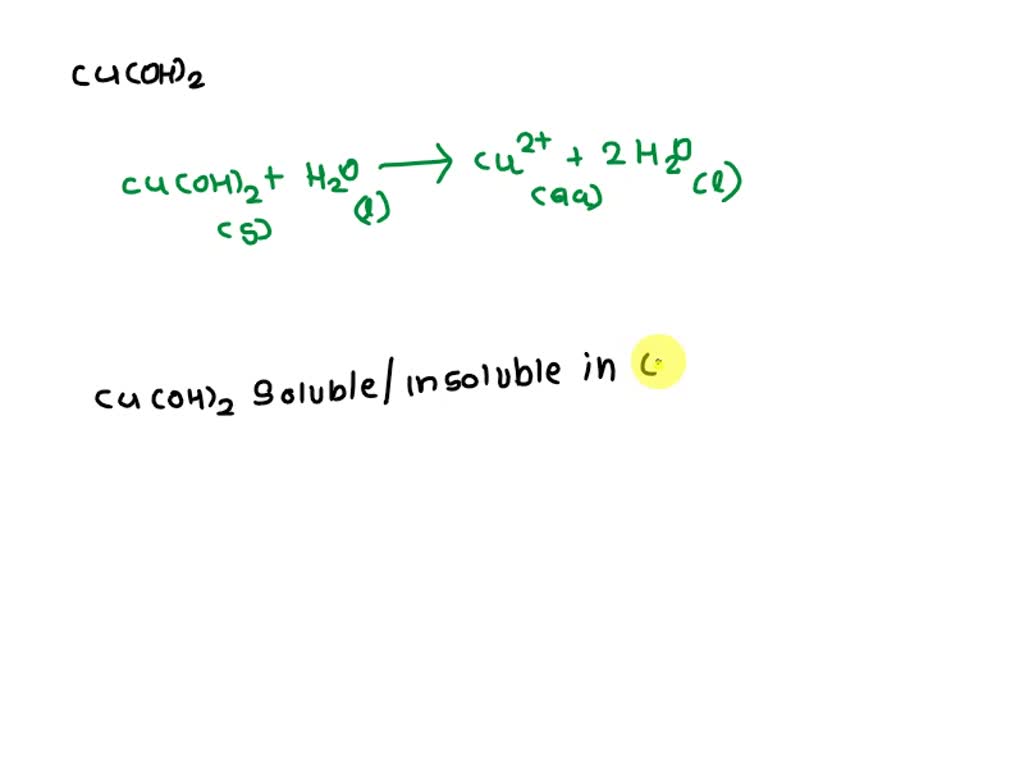
Copper Hydroxide Dissolve In Water . Most metal hydroxides are insoluble; This value was obtained in the course of a study of the. It decomposes at temperatures above 185. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. More importantly, it will dissolve freely and act as. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. To learn more about the structure, physical properties, chemical. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. It is slightly soluble in water and more soluble in acids or ammonium hydroxide.
from www.numerade.com
To learn more about the structure, physical properties, chemical. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. It decomposes at temperatures above 185. More importantly, it will dissolve freely and act as. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. This value was obtained in the course of a study of the. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Most metal hydroxides are insoluble;
SOLVED A precipitate of copper(II) hydroxide is formed in a reaction
Copper Hydroxide Dissolve In Water Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. More importantly, it will dissolve freely and act as. Most metal hydroxides are insoluble; It decomposes at temperatures above 185. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. To learn more about the structure, physical properties, chemical. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. This value was obtained in the course of a study of the.
From fphoto.photoshelter.com
science chemistry precipitation reaction cupric hydroxide Fundamental Copper Hydroxide Dissolve In Water It decomposes at temperatures above 185. To learn more about the structure, physical properties, chemical. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. More importantly, it will dissolve freely and act as. Most metal hydroxides are insoluble; It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper hydroxide reacts with sulfuric. Copper Hydroxide Dissolve In Water.
From klalixjsm.blob.core.windows.net
Copper Hydroxide Plus Sulfuric Acid at Mildred Anderson blog Copper Hydroxide Dissolve In Water This value was obtained in the course of a study of the. More importantly, it will dissolve freely and act as. It decomposes at temperatures above 185. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. Copper hydroxide reacts with sulfuric acid to. Copper Hydroxide Dissolve In Water.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Hydroxide Dissolve In Water Most metal hydroxides are insoluble; More importantly, it will dissolve freely and act as. To learn more about the structure, physical properties, chemical. It decomposes at temperatures above 185. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Some such as ca(oh). Copper Hydroxide Dissolve In Water.
From digital-analysis.com
Heavy Metal Reduction for Industrial Wastewater Copper Hydroxide Dissolve In Water Most metal hydroxides are insoluble; This value was obtained in the course of a study of the. More importantly, it will dissolve freely and act as. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. To learn more about the structure, physical. Copper Hydroxide Dissolve In Water.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Dissolve In Water It decomposes at temperatures above 185. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Most metal hydroxides are insoluble; To learn more about the structure, physical properties, chemical. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. More. Copper Hydroxide Dissolve In Water.
From www.youtube.com
Reaction of Calcium Hydroxide and Copper Sulfate in Water YouTube Copper Hydroxide Dissolve In Water To learn more about the structure, physical properties, chemical. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It decomposes at temperatures above 185. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. More importantly, it will. Copper Hydroxide Dissolve In Water.
From fineartamerica.com
Hydroxide Precipitates Photograph by Andrew Lambert Photography Copper Hydroxide Dissolve In Water Most metal hydroxides are insoluble; To learn more about the structure, physical properties, chemical. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. It decomposes at temperatures above 185. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It is slightly soluble in water and more soluble in acids or ammonium. Copper Hydroxide Dissolve In Water.
From www.youtube.com
Copper(II) Sulfate and Sodium Hydroxide reaction CuSO4 + NaOH Copper Hydroxide Dissolve In Water It decomposes at temperatures above 185. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. More importantly, it will dissolve freely and act as. To learn more about. Copper Hydroxide Dissolve In Water.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Dissolve In Water To learn more about the structure, physical properties, chemical. More importantly, it will dissolve freely and act as. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Most metal hydroxides are insoluble; This value was obtained in the course of. Copper Hydroxide Dissolve In Water.
From www.youtube.com
Making copper hydroxide YouTube Copper Hydroxide Dissolve In Water Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. This value was obtained in the course of a study of the. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. More importantly, it will dissolve freely and act as. To learn more about the structure, physical properties, chemical. It decomposes at. Copper Hydroxide Dissolve In Water.
From www.youtube.com
Reaction between copper(II)sulfate and potassium hydroxide YouTube Copper Hydroxide Dissolve In Water It decomposes at temperatures above 185. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Most metal hydroxides are insoluble; This value was obtained in the course of a study of the. To learn more about the structure, physical properties, chemical. Some such. Copper Hydroxide Dissolve In Water.
From www.youtube.com
Copper Hydroxide Synthesis YouTube Copper Hydroxide Dissolve In Water More importantly, it will dissolve freely and act as. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. It decomposes at temperatures above 185. Most metal hydroxides are insoluble; To learn. Copper Hydroxide Dissolve In Water.
From www.sciencephoto.com
Copper Hydroxide Precipitate Stock Image C027/9450 Science Photo Copper Hydroxide Dissolve In Water Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. This value was obtained in the course of a study of the. More importantly, it will dissolve freely and act as. To learn more about the structure, physical properties, chemical. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. It decomposes at temperatures above 185.. Copper Hydroxide Dissolve In Water.
From www.sciencephoto.com
Copper Hydroxide Precipitate Stock Image C027/9441 Science Photo Copper Hydroxide Dissolve In Water Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. Most metal hydroxides are insoluble; More importantly, it will dissolve freely and act as. It decomposes at temperatures above 185. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. This. Copper Hydroxide Dissolve In Water.
From www.flickr.com
Copper hydroxide Copper hydroxide (Cu(OH)2) suspended in s… Flickr Copper Hydroxide Dissolve In Water It decomposes at temperatures above 185. Most metal hydroxides are insoluble; It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. To learn more about the structure, physical properties,. Copper Hydroxide Dissolve In Water.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Dissolve In Water Most metal hydroxides are insoluble; It is slightly soluble in water and more soluble in acids or ammonium hydroxide. More importantly, it will dissolve freely and act as. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. To learn more about the structure, physical properties, chemical. This value was obtained in the course of. Copper Hydroxide Dissolve In Water.
From woelen.homescience.net
Science made alive Chemistry/Experiments Copper Hydroxide Dissolve In Water This value was obtained in the course of a study of the. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. More importantly, it will dissolve freely and act as. To learn more about the structure, physical properties, chemical. It. Copper Hydroxide Dissolve In Water.
From www.youtube.com
Copper complex formation Copper hydroxide and ammonia MVI 6587 YouTube Copper Hydroxide Dissolve In Water It decomposes at temperatures above 185. To learn more about the structure, physical properties, chemical. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. This value was obtained. Copper Hydroxide Dissolve In Water.
From www.numerade.com
SOLVED A precipitate of copper(II) hydroxide is formed in a reaction Copper Hydroxide Dissolve In Water To learn more about the structure, physical properties, chemical. This value was obtained in the course of a study of the. Most metal hydroxides are insoluble; It decomposes at temperatures above 185. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. More importantly,. Copper Hydroxide Dissolve In Water.
From www.sciencephoto.com
Copper Hydroxide Precipitate, 2 of 3 Stock Image C030/8095 Copper Hydroxide Dissolve In Water This value was obtained in the course of a study of the. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It decomposes at temperatures above 185. Copper. Copper Hydroxide Dissolve In Water.
From fineartamerica.com
Hydroxide Precipitates Photograph by Andrew Lambert Photography Fine Copper Hydroxide Dissolve In Water It is slightly soluble in water and more soluble in acids or ammonium hydroxide. More importantly, it will dissolve freely and act as. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. It decomposes at temperatures above 185. This value was obtained in the course of a study of the. To learn more about the structure, physical. Copper Hydroxide Dissolve In Water.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide Dissolve In Water More importantly, it will dissolve freely and act as. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. This value was obtained in the course of a study of the.. Copper Hydroxide Dissolve In Water.
From joiztoyae.blob.core.windows.net
Copper Hydroxide Soluble In Water at Walter Stanley blog Copper Hydroxide Dissolve In Water Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. It decomposes at temperatures above 185. More importantly, it will dissolve freely and act as. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. This value was obtained in the course of a study of the. Copper (ii) hydroxide has some small solubility in water,. Copper Hydroxide Dissolve In Water.
From fineartamerica.com
Precipitation Of Copper (ii) Hydroxide Photograph by David Taylor Copper Hydroxide Dissolve In Water This value was obtained in the course of a study of the. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. To learn more about the structure, physical properties, chemical. Most metal hydroxides are insoluble; It is slightly soluble in water and more soluble in acids or ammonium hydroxide. More importantly, it will dissolve. Copper Hydroxide Dissolve In Water.
From socratic.org
How do you know when copper(II) sulfate hydrated water evaporates Copper Hydroxide Dissolve In Water It decomposes at temperatures above 185. Most metal hydroxides are insoluble; More importantly, it will dissolve freely and act as. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. To learn more about the structure, physical properties, chemical. This value was obtained in the course of a study of the. Copper (ii) hydroxide has some small solubility. Copper Hydroxide Dissolve In Water.
From www.youtube.com
DIY Making Copper Hydroxide via Electrolysis YouTube Copper Hydroxide Dissolve In Water Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. To learn more about the structure, physical properties, chemical. It decomposes at temperatures above 185. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Most metal hydroxides are. Copper Hydroxide Dissolve In Water.
From pixels.com
Precipitation Of Copper (ii) Hydroxide Photograph by David Taylor Copper Hydroxide Dissolve In Water Most metal hydroxides are insoluble; This value was obtained in the course of a study of the. It decomposes at temperatures above 185. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper (ii) hydroxide has some small solubility in water, determined by. Copper Hydroxide Dissolve In Water.
From www.youtube.com
Precipitation Reactions Copper Hydroxide Junior YouTube Copper Hydroxide Dissolve In Water It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. It decomposes at temperatures above 185. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. To learn more about the structure, physical properties, chemical. This value was obtained in the course. Copper Hydroxide Dissolve In Water.
From ar.inspiredpencil.com
Copper Hydroxide Solution Copper Hydroxide Dissolve In Water Most metal hydroxides are insoluble; It decomposes at temperatures above 185. More importantly, it will dissolve freely and act as. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. This value. Copper Hydroxide Dissolve In Water.
From fineartamerica.com
Copper Hydroxide Precipitate Photograph by Andrew Lambert Photography Copper Hydroxide Dissolve In Water It decomposes at temperatures above 185. Most metal hydroxides are insoluble; This value was obtained in the course of a study of the. To learn more about the structure, physical properties, chemical. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. More importantly, it will dissolve freely and act as. It is slightly soluble in water and. Copper Hydroxide Dissolve In Water.
From woelen.homescience.net
Science made alive Chemistry/Experiments Copper Hydroxide Dissolve In Water This value was obtained in the course of a study of the. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Most metal hydroxides are insoluble; To learn more about the structure, physical properties, chemical. More importantly, it will dissolve. Copper Hydroxide Dissolve In Water.
From www.sciencephoto.com
Copper hydroxide Stock Image C029/9630 Science Photo Library Copper Hydroxide Dissolve In Water This value was obtained in the course of a study of the. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. More importantly, it will dissolve freely and act as. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant.. Copper Hydroxide Dissolve In Water.
From ar.inspiredpencil.com
Copper Hydroxide Solution Copper Hydroxide Dissolve In Water Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. This value was obtained in the course of a study of the. To learn more about the structure, physical properties, chemical. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. More. Copper Hydroxide Dissolve In Water.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper Hydroxide Dissolve In Water To learn more about the structure, physical properties, chemical. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. Some such as ca(oh) 2, mg(oh) 2, fe(oh) 2, al(oh) 3 etc. This value was obtained in the course of a study of the. More importantly, it will dissolve freely and act as. Copper hydroxide reacts with. Copper Hydroxide Dissolve In Water.
From www.youtube.com
Make copper hydroxide with just two compounds YouTube Copper Hydroxide Dissolve In Water Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. More importantly, it will dissolve freely and act as. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. It is slightly soluble in water and more soluble in acids or ammonium hydroxide. It decomposes at temperatures above 185. To learn more about. Copper Hydroxide Dissolve In Water.