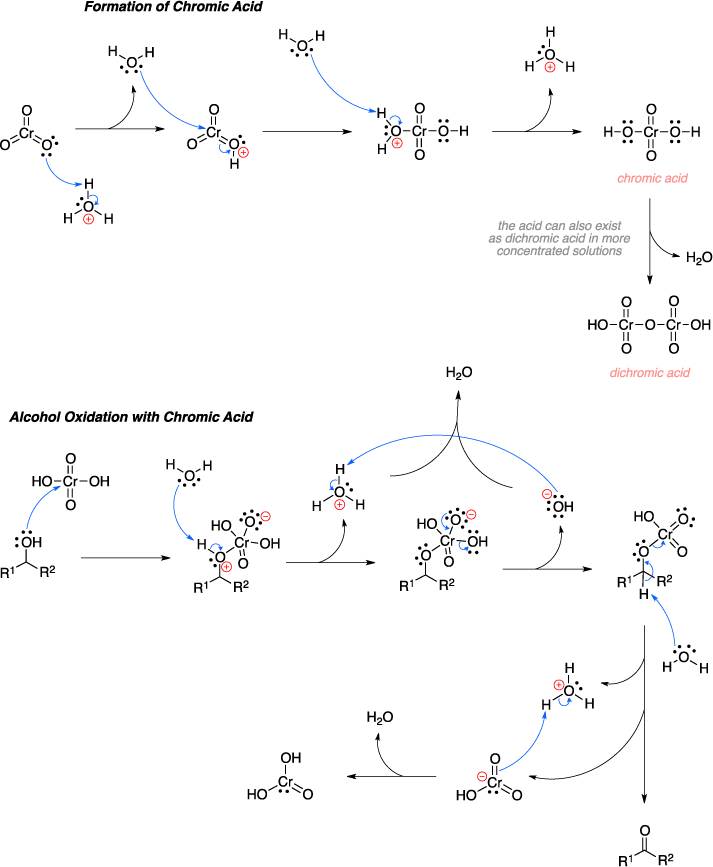
Jones Reagent Organic Chemistry . chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. There is a wide selection of oxidizing. The jones oxidation also uses acetone as a co. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the oxidizing agent.
from www.name-reaction.com
the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. The jones oxidation also uses acetone as a co. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the oxidizing agent. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. There is a wide selection of oxidizing.
Jones oxidation
Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the oxidizing agent. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. The jones oxidation also uses acetone as a co. There is a wide selection of oxidizing. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid.
From www.chegg.com
Solved 8439026 2 / 7 1. Predict the major organic product Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. The jones oxidation also uses acetone as a co. a common method for oxidizing secondary alcohols to ketones. Jones Reagent Organic Chemistry.
From www.youtube.com
Oxidation of Alcohols by PCC + Jones Reagent H2CrO4 (Whiteboard Strikes Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. The jones oxidation also uses acetone as a co. the jones reagent is a solution of chromium trioxide. Jones Reagent Organic Chemistry.
From www.synarchive.com
Jones Oxidation Jones Reagent Organic Chemistry The jones oxidation also uses acetone as a co. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. There is a wide selection of oxidizing. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic.. Jones Reagent Organic Chemistry.
From www.studypool.com
SOLUTION Concise organic chemistry reagent and reaction guide Studypool Jones Reagent Organic Chemistry the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as. Jones Reagent Organic Chemistry.
From www.researchgate.net
Scheme 8 Reagents and conditions i) 10 KOH, MeOH; ii) Jones' reagent Jones Reagent Organic Chemistry the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. There is a wide selection of oxidizing. chromic acid, also known as jones reagent, is. Jones Reagent Organic Chemistry.
From www.chemistrysteps.com
Organic Chemistry Reagent Guide Chemistry Steps Jones Reagent Organic Chemistry the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be. Jones Reagent Organic Chemistry.
From www.masterorganicchemistry.com
The Organic Chemistry Reagent Guide is here! Master Organic Chemistry Jones Reagent Organic Chemistry The jones oxidation also uses acetone as a co. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones reagent is a mixture of chromic trioxide or. Jones Reagent Organic Chemistry.
From www.youtube.com
Jones reagent H2CrO4 oxidation chemistry Advanced Organic reactions Jones Reagent Organic Chemistry the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the oxidizing agent. There is a wide selection of oxidizing. the jones reagent is a mixture of chromic trioxide or sodium dichromate in. Jones Reagent Organic Chemistry.
From www.name-reaction.com
Jones oxidation Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the oxidizing agent. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in.. Jones Reagent Organic Chemistry.
From www.youtube.com
Collins Reagent,Jones Reagent10 minute ReagentCSIR NET/GATE/IIT JAM Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. The jones oxidation also uses acetone as a co. There is a wide selection of oxidizing. chromic acid,. Jones Reagent Organic Chemistry.
From www.youtube.com
Oxidation Jones Reagent Mechanism YouTube Jones Reagent Organic Chemistry The jones oxidation also uses acetone as a co. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. the jones oxidation is an organic reaction for. Jones Reagent Organic Chemistry.
From studylib.net
Jones Test Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the oxidizing agent. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of. Jones Reagent Organic Chemistry.
From www.youtube.com
Jones Reagent Part1 Reagent Series YouTube Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. The jones oxidation also uses acetone as a co. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. the jones reagent is a solution of chromium trioxide. Jones Reagent Organic Chemistry.
From chemistry-reaction.com
List of Common Reagents Used in Organic Chemistry « Organic Chemistry Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic. Jones Reagent Organic Chemistry.
From getcheatsheet.blogspot.com
Organic Chemistry Reagents Cheat Sheet Cheat Sheet Jones Reagent Organic Chemistry The jones oxidation also uses acetone as a co. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. a common method for oxidizing secondary alcohols. Jones Reagent Organic Chemistry.
From www.youtube.com
Jones Oxidation Named Reactions Organic Chemistry Lessons YouTube Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the oxidizing agent. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the. Jones Reagent Organic Chemistry.
From www.masterorganicchemistry.com
What Are Reducing Sugars? Master Organic Chemistry Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the oxidizing agent. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. The. Jones Reagent Organic Chemistry.
From www.researchgate.net
Oxidation of Benzoins with the Jones Reagent Supported on Kieselguhr Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for. Jones Reagent Organic Chemistry.
From www.chemistrylearner.com
Jones Reagent Definition, Preparation, and Mechanism. Jones Reagent Organic Chemistry the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the oxidizing agent. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid.. Jones Reagent Organic Chemistry.
From www.pinterest.com
[77] Jones Oxidation 1946 Organic chemistry study, Organic chemistry Jones Reagent Organic Chemistry the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro. Jones Reagent Organic Chemistry.
From www.pw.live
Jones reagent Reaction Mechanism of Jone’s reagent Jones Reagent Organic Chemistry There is a wide selection of oxidizing. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. The jones oxidation also uses acetone as a co. . Jones Reagent Organic Chemistry.
From www.chegg.com
Solved Organic Chemistry II Chapter 11 Oxidation Practice Jones Reagent Organic Chemistry the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the. Jones Reagent Organic Chemistry.
From www.youtube.com
Synthesis Using Jones Reagent Organic Chemistry YouTube Jones Reagent Organic Chemistry There is a wide selection of oxidizing. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. The jones oxidation also uses acetone as a co.. Jones Reagent Organic Chemistry.
From www.youtube.com
Oxidation of Alcohols Using Jones Reagent and PCC YouTube Jones Reagent Organic Chemistry the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to. Jones Reagent Organic Chemistry.
From www.youtube.com
Jones Reagent Vs PCC Oxidation Organic Chemistry youtubeshorts Jones Reagent Organic Chemistry the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3). Jones Reagent Organic Chemistry.
From chemistry.stackexchange.com
organic chemistry Usage of TFA in PfitznerMoffat oxidation and Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic. Jones Reagent Organic Chemistry.
From www.youtube.com
Chemoselective ReagentsAlcohol OxidationPCC/PDC,Jones Oxidation Jones Reagent Organic Chemistry the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to. Jones Reagent Organic Chemistry.
From www.studypool.com
SOLUTION Organic chemistry reagent guide Studypool Jones Reagent Organic Chemistry There is a wide selection of oxidizing. The jones oxidation also uses acetone as a co. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. chromic. Jones Reagent Organic Chemistry.
From chem.libretexts.org
Oxidation by Chromic Acid Chemistry LibreTexts Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. a common method for oxidizing secondary alcohols to ketones uses chromic acid (h2cro4) as the oxidizing agent. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. The. Jones Reagent Organic Chemistry.
From www.youtube.com
Oxidation Organic Chemistry Jones Reagent and PCC YouTube Jones Reagent Organic Chemistry the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely. Jones Reagent Organic Chemistry.
From www.pinterest.com
Pin on Chemistry Jones Reagent Organic Chemistry the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms chromic acid in. There is a wide selection of oxidizing. The jones oxidation also uses acetone as a co. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the. Jones Reagent Organic Chemistry.
From www.studypool.com
SOLUTION organic chemistry reagent guide Studypool Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. There is a wide selection of oxidizing. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. chromic acid, also known as jones reagent, is prepared. Jones Reagent Organic Chemistry.
From www.pinterest.com.mx
organic chemistry reagents Organic chemistry, Organic chemistry study Jones Reagent Organic Chemistry The jones oxidation also uses acetone as a co. There is a wide selection of oxidizing. chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. . Jones Reagent Organic Chemistry.
From mungfali.com
Organic Chemistry Reagent Chart Jones Reagent Organic Chemistry the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. the jones reagent is a solution of chromium trioxide in diluted sulfuric acid that can be used safely for oxidations of organic. The jones oxidation also uses acetone as a co. the jones reagent is a mixture. Jones Reagent Organic Chemistry.
From www.thecarbondr.com
Oxidation Reactions in Organic Chemistry Alcohols Jones Reagent Organic Chemistry chromic acid, also known as jones reagent, is prepared by adding chromium trioxide (cro 3) to aqueous sulfuric acid. the jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones,. the jones reagent is a mixture of chromic trioxide or sodium dichromate in diluted sulfuric acid, which forms. Jones Reagent Organic Chemistry.