Magnesium Ion And Chloride Ion . magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. thus, the ratio of magnesium ion: a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: 2 and the simplest magnesium chloride chemical formula is. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: It has a melting point of 714. Mg 2+ cl − combining one ion of each does not. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. Mg 2 + cl − combining one ion of each does not completely. See the balanced chemical equation, the atomic structure and.
from www.benjamin-mills.com
a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: thus, the ratio of magnesium ion: a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). It has a melting point of 714. magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. Mg 2+ cl − combining one ion of each does not. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. 2 and the simplest magnesium chloride chemical formula is.
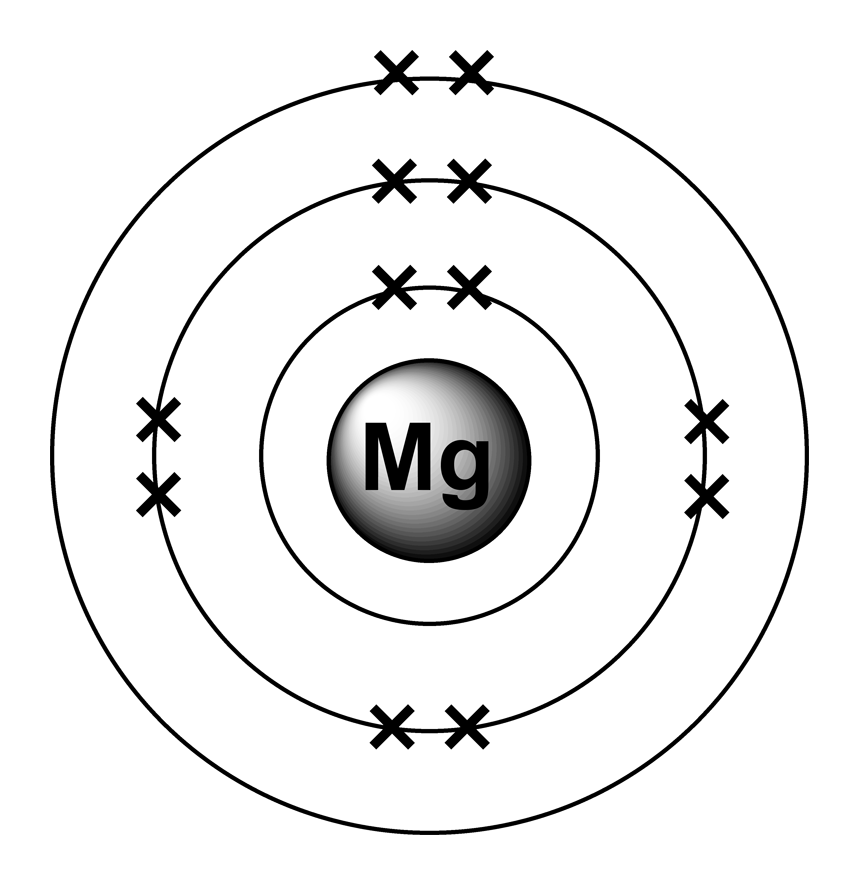
Electron configurations
Magnesium Ion And Chloride Ion to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg 2 + cl − combining one ion of each does not completely. See the balanced chemical equation, the atomic structure and. Mg 2+ cl − combining one ion of each does not. It has a melting point of 714. thus, the ratio of magnesium ion: to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). 2 and the simplest magnesium chloride chemical formula is. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive.
From oneclass.com
OneClass 3. Consider the magnesium ion (Mg) and the chloride anion (Ch Magnesium Ion And Chloride Ion a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. a magnesium ion has a 2+ charge, while a chlorine ion. Magnesium Ion And Chloride Ion.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Ion And Chloride Ion thus, the ratio of magnesium ion: learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). It has a melting point of 714. See the balanced chemical equation, the atomic structure and. magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. to balance the charges with the. Magnesium Ion And Chloride Ion.
From brainly.in
[Answered] show the formation of magnesium chloride from magnesium and Magnesium Ion And Chloride Ion thus, the ratio of magnesium ion: It has a melting point of 714. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: See the balanced chemical equation, the atomic structure and. Mg 2 + cl − combining one ion of each does not completely. magnesium chloride (mgcl2) is an ionic compound. Magnesium Ion And Chloride Ion.
From diagramdataperianths.z21.web.core.windows.net
Diagram Ionic Bond Magnesium Ion And Chloride Ion See the balanced chemical equation, the atomic structure and. It has a melting point of 714. Mg 2+ cl − combining one ion of each does not. learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). to balance the charges with the lowest number of ions possible, we need to have two chloride. Magnesium Ion And Chloride Ion.
From userdatabespangles.z14.web.core.windows.net
Magnesium Atomic Structure Diagram Magnesium Ion And Chloride Ion learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. thus, the ratio of magnesium ion: 2 and the simplest magnesium chloride chemical formula is. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg 2+ cl − combining one. Magnesium Ion And Chloride Ion.
From socratic.org
Question 15936 + Example Magnesium Ion And Chloride Ion See the balanced chemical equation, the atomic structure and. Mg 2+ cl − combining one ion of each does not. Mg 2 + cl − combining one ion of each does not completely. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. thus, the ratio of. Magnesium Ion And Chloride Ion.
From userdatalicensures.z5.web.core.windows.net
Lewis Dot Diagram For Chloride Ion Magnesium Ion And Chloride Ion Mg 2 + cl − combining one ion of each does not completely. thus, the ratio of magnesium ion: It has a melting point of 714. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Magnesium Ion And Chloride Ion.
From quizizz.com
Properties of Ionic Compounds Chemistry Quiz Quizizz Magnesium Ion And Chloride Ion a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg 2+ cl − combining one ion of each does not. Mg 2 + cl − combining one ion of each does not completely. magnesium chloride (mgcl2). Magnesium Ion And Chloride Ion.
From brainly.in
Write the formation of magnesium chloride (MgCl,) with the help of Magnesium Ion And Chloride Ion It has a melting point of 714. 2 and the simplest magnesium chloride chemical formula is. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. Mg 2+ cl − combining one ion of each does not. Mg 2. Magnesium Ion And Chloride Ion.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion And Chloride Ion magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. 2 and the simplest magnesium chloride chemical formula is. Mg 2+ cl − combining one ion of each does not. thus, the ratio of magnesium ion: See the balanced chemical equation, the atomic structure and. learn how to write the chemical formula for. Magnesium Ion And Chloride Ion.
From www.elektramagnesium.com.au
Ionic Magnesium Chloride Hexahydrate or Chelated Magnesium? Elektra Magnesium Ion And Chloride Ion Mg 2+ cl − combining one ion of each does not. See the balanced chemical equation, the atomic structure and. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Magnesium Ion And Chloride Ion.
From www.vecteezy.com
Magnesium symbol. Chemical element of the periodic table. Vector Magnesium Ion And Chloride Ion magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). See the balanced chemical equation, the atomic structure and. It has a melting point of 714. 2 and the simplest magnesium chloride chemical formula is. magnesium chloride (mgcl2) is. Magnesium Ion And Chloride Ion.
From ar.inspiredpencil.com
Magnesium Ion Lewis Dot Structure Magnesium Ion And Chloride Ion See the balanced chemical equation, the atomic structure and. Mg 2 + cl − combining one ion of each does not completely. Mg 2+ cl − combining one ion of each does not. It has a melting point of 714. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. a magnesium. Magnesium Ion And Chloride Ion.
From galvinconanstuart.blogspot.com
Dot Diagram Of Magnesium Chloride General Wiring Diagram Magnesium Ion And Chloride Ion magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). It has a melting point of 714. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge. Magnesium Ion And Chloride Ion.
From mungfali.com
PPT Section 9.1 Naming Ions PowerPoint Presentation Magnesium Ion And Chloride Ion learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg 2 + cl − combining one ion of each does not completely. thus, the ratio of magnesium ion: magnesium. Magnesium Ion And Chloride Ion.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Ion And Chloride Ion See the balanced chemical equation, the atomic structure and. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: magnesium chloride (mgcl2) is a chemical compound with one magnesium and two. Magnesium Ion And Chloride Ion.
From cebhjyoy.blob.core.windows.net
Chlorine Has 7 Valence Electrons. Why Would It Love To Gain Another Magnesium Ion And Chloride Ion a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. 2 and the simplest magnesium chloride chemical formula is. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing. Magnesium Ion And Chloride Ion.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Library Magnesium Ion And Chloride Ion It has a melting point of 714. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. thus, the ratio of magnesium ion: Mg 2+ cl − combining one. Magnesium Ion And Chloride Ion.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Ion And Chloride Ion to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. Mg 2 + cl − combining one ion of each does not completely. 2 and the simplest magnesium chloride chemical formula is. magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride. Magnesium Ion And Chloride Ion.
From userdatalicensures.z5.web.core.windows.net
Lewis Dot Diagrams Showing Bonding Magnesium Ion And Chloride Ion learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). Mg 2+ cl − combining one ion of each does not. It has a melting point of 714. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. magnesium chloride (mgcl2) is. Magnesium Ion And Chloride Ion.
From stock.adobe.com
Set of blue electrolyte modern icons Calcium, Sodium, Magnesium Magnesium Ion And Chloride Ion 2 and the simplest magnesium chloride chemical formula is. learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). It has a melting point of 714. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. magnesium chloride (mgcl2) is. Magnesium Ion And Chloride Ion.
From userdatabespangles.z14.web.core.windows.net
Lewis Dot Diagram For Magnesium Chloride Magnesium Ion And Chloride Ion 2 and the simplest magnesium chloride chemical formula is. Mg 2 + cl − combining one ion of each does not completely. magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. Mg 2+ cl − combining one ion of each does not. to balance the charges with the lowest number of ions possible,. Magnesium Ion And Chloride Ion.
From www.benjamin-mills.com
Electron configurations Magnesium Ion And Chloride Ion a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg 2 + cl − combining one ion of each does not completely. thus, the ratio of magnesium ion: magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. learn how magnesium and chlorine form. Magnesium Ion And Chloride Ion.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Magnesium Ion And Chloride Ion See the balanced chemical equation, the atomic structure and. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. Mg 2+ cl − combining one ion of each does not. learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). 2 and the simplest magnesium chloride chemical formula. Magnesium Ion And Chloride Ion.
From keystagewiki.com
Giant Ionic Structure Key Stage Wiki Magnesium Ion And Chloride Ion learn how magnesium and chlorine form an ionic bond to make magnesium chloride (mgcl2). See the balanced chemical equation, the atomic structure and. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: 2 and the simplest magnesium chloride chemical formula is. It has a melting point of 714. learn how to. Magnesium Ion And Chloride Ion.
From mydiagram.online
[DIAGRAM] Lewis Electron Dot Diagrams Magnesium Magnesium Ion And Chloride Ion learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. Mg 2+ cl − combining one ion of each does not. It has a melting point of 714. Mg 2 + cl − combining one ion of each does not completely. thus, the ratio of magnesium ion:. Magnesium Ion And Chloride Ion.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Ion And Chloride Ion a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg 2+ cl − combining one ion of each does not. Mg 2 + cl − combining one ion of each does not completely. It has a melting point of 714. learn how to write the chemical formula for a simple ionic compound,. Magnesium Ion And Chloride Ion.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Magnesium Ion And Chloride Ion 2 and the simplest magnesium chloride chemical formula is. Mg 2 + cl − combining one ion of each does not completely. It has a melting point of 714. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. learn how to write the chemical formula for a simple ionic compound, such. Magnesium Ion And Chloride Ion.
From www.pngwing.com
Lewis structure Magnesium chloride Electron Diagram, Dot, text Magnesium Ion And Chloride Ion a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: It has a melting. Magnesium Ion And Chloride Ion.
From brainly.in
I) show the formation of magnesium chloride and sodium chloride by Magnesium Ion And Chloride Ion Mg 2+ cl − combining one ion of each does not. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. thus, the ratio of magnesium ion: a magnesium ion has a 2+ charge, while a. Magnesium Ion And Chloride Ion.
From circuitdiagramroutine.z14.web.core.windows.net
Dot And Cross Diagram For Magnesium Chloride Magnesium Ion And Chloride Ion 2 and the simplest magnesium chloride chemical formula is. thus, the ratio of magnesium ion: learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. learn how magnesium and. Magnesium Ion And Chloride Ion.
From wirelistsightsmen.z21.web.core.windows.net
Lewis Dot Diagram For Magnesium Oxide Magnesium Ion And Chloride Ion magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. Mg 2+ cl − combining one. Magnesium Ion And Chloride Ion.
From socialmediaposting.blogspot.com
MAGNESIUM CHLORIDE BENEFITS Viral Media Magnesium Ion And Chloride Ion Mg 2+ cl − combining one ion of each does not. Mg 2 + cl − combining one ion of each does not completely. magnesium chloride (mgcl2) is a chemical compound with one magnesium and two chloride ions. See the balanced chemical equation, the atomic structure and. a magnesium ion has a 2+ charge, while a chlorine ion. Magnesium Ion And Chloride Ion.
From byjus.com
By the transfer of electrons, illustrate the formation of bond in Magnesium Ion And Chloride Ion Mg 2+ cl − combining one ion of each does not. 2 and the simplest magnesium chloride chemical formula is. magnesium chloride (mgcl2) is an ionic compound composed of one magnesium ion and two chloride ions. Mg 2 + cl − combining one ion of each does not completely. thus, the ratio of magnesium ion: learn how. Magnesium Ion And Chloride Ion.
From circuitdiagramlevee.z21.web.core.windows.net
Lewis Dot Diagram For Magnesium And Chlorine Magnesium Ion And Chloride Ion Mg 2+ cl − combining one ion of each does not. 2 and the simplest magnesium chloride chemical formula is. It has a melting point of 714. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: See the balanced chemical equation, the atomic structure and. learn how magnesium and chlorine form an. Magnesium Ion And Chloride Ion.