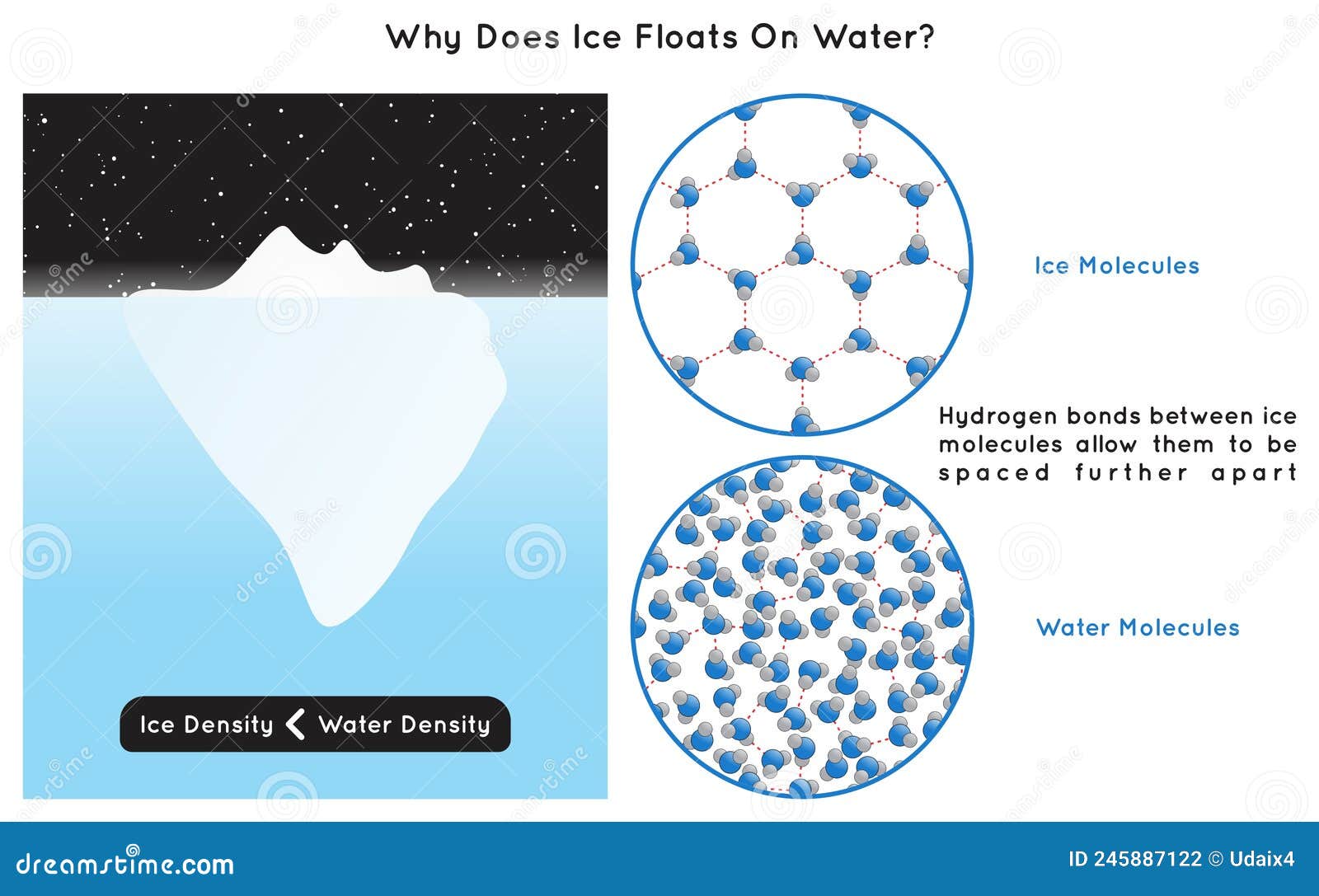
Why Do Ice Float On Water Class 9 . why does ice float on water. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. ice floats because it is about 9% less dense than liquid water. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. Why ice floats on water? discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. In other words, ice takes up about 9% more space than water, so a liter of ice. ice cubes float because of their molecular structure. The hydrogen and oxygen atoms share electron pairs, forming. Ice has a highly ordered three. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice is crystalline form of water: why do we see water droplets collected on the outer surface of a glass container, containing ice?
from www.dreamstime.com
Why ice floats on water? Ice is crystalline form of water: In other words, ice takes up about 9% more space than water, so a liter of ice. ice cubes float because of their molecular structure. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. why does ice float on water. why do we see water droplets collected on the outer surface of a glass container, containing ice?
Why Does Ice Float on Water Infographic Diagram Stock Vector
Why Do Ice Float On Water Class 9 It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. In other words, ice takes up about 9% more space than water, so a liter of ice. ice cubes float because of their molecular structure. The hydrogen and oxygen atoms share electron pairs, forming. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. why does ice float on water. Ice has a highly ordered three. ice floats because it is about 9% less dense than liquid water. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. Ice is crystalline form of water: why do we see water droplets collected on the outer surface of a glass container, containing ice? Why ice floats on water?
From www.youtube.com
Liquids generally have lower density compared to solid but you have Why Do Ice Float On Water Class 9 Ice has a highly ordered three. Why ice floats on water? discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. In other words, ice takes up about 9% more space than water, so a liter of ice. why does ice float on water. at zero degrees,. Why Do Ice Float On Water Class 9.
From www.youtube.com
Why Ice Floats on Water YouTube Why Do Ice Float On Water Class 9 The hydrogen and oxygen atoms share electron pairs, forming. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. ice cubes float because of their molecular structure. Ice is crystalline form of water: why do we see water droplets collected on the outer surface of. Why Do Ice Float On Water Class 9.
From www.pitara.com
How Does Ice float? Pitara Kids' Network Why Do Ice Float On Water Class 9 Ice is crystalline form of water: ice floats because it is about 9% less dense than liquid water. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. why do we see water droplets collected on the outer surface of a glass container, containing ice? at. Why Do Ice Float On Water Class 9.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector Why Do Ice Float On Water Class 9 Ice is crystalline form of water: A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. why do we see water droplets collected on the outer surface of a glass container, containing ice? Why ice floats on water? ice cubes float because of their molecular structure. at zero degrees, i.e., the temperature at. Why Do Ice Float On Water Class 9.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Do Ice Float On Water Class 9 Ice is crystalline form of water: why does ice float on water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. why do we see water droplets collected on the outer surface of a glass container, containing ice? Why ice floats on water? In other words, ice takes up about 9% more space. Why Do Ice Float On Water Class 9.
From www.jagranjosh.com
What is the reason behind floating ice on water Why Do Ice Float On Water Class 9 Why ice floats on water? A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice is crystalline form of water: at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. discover the unusual properties of water that make ice less dense and how. Why Do Ice Float On Water Class 9.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare Why Do Ice Float On Water Class 9 A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. ice floats because it is about 9% less dense than liquid water. The hydrogen and oxygen atoms share electron pairs, forming. Ice has a highly ordered three. why does ice float on water. why do we see water droplets collected on the outer. Why Do Ice Float On Water Class 9.
From www.slideserve.com
PPT Water, water everywhere! PowerPoint Presentation, free download Why Do Ice Float On Water Class 9 It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. why do we see water droplets collected on the outer surface of a glass container, containing ice? A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. at zero degrees, i.e.,. Why Do Ice Float On Water Class 9.
From www.branchor.com
Why does ice float on water? Exploring the science and consequences Why Do Ice Float On Water Class 9 In other words, ice takes up about 9% more space than water, so a liter of ice. ice cubes float because of their molecular structure. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. A water molecule (h2o) is made of two hydrogen atoms and one oxygen. Why Do Ice Float On Water Class 9.
From www.slideserve.com
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download Why Do Ice Float On Water Class 9 It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. In other words, ice takes up about 9% more space than water, so a liter of ice. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite. Why Do Ice Float On Water Class 9.
From studylib.net
Why Does Ice Float In Water And Not In Alcohol Why Do Ice Float On Water Class 9 why does ice float on water. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. ice cubes float because of their molecular structure. In other words, ice takes up about 9% more space than water, so a liter of ice. Ice is crystalline form. Why Do Ice Float On Water Class 9.
From www.youtube.com
Why do ice floats on water class 11? YouTube Why Do Ice Float On Water Class 9 The hydrogen and oxygen atoms share electron pairs, forming. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. discover the unusual properties of water that make ice less dense and how this affects. Why Do Ice Float On Water Class 9.
From www.youtube.com
Why do ice float on water but sink in alcohol? YouTube Why Do Ice Float On Water Class 9 A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice is crystalline form of water: ice floats because it is about 9% less dense than liquid water. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. Ice has a highly ordered three.. Why Do Ice Float On Water Class 9.
From www.youtube.com
Why ICE FLOATS on WATER YouTube Why Do Ice Float On Water Class 9 why do we see water droplets collected on the outer surface of a glass container, containing ice? A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Why ice floats on water? discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. at. Why Do Ice Float On Water Class 9.
From www.youtube.com
Why does ice float on water? Detailed Explanation YouTube Why Do Ice Float On Water Class 9 ice cubes float because of their molecular structure. why do we see water droplets collected on the outer surface of a glass container, containing ice? at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. Ice is crystalline form of water: Ice has a highly ordered three.. Why Do Ice Float On Water Class 9.
From brainly.in
Why does ice float on water even if it is a solid Brainly.in Why Do Ice Float On Water Class 9 ice cubes float because of their molecular structure. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. why do we see water droplets collected on the outer surface of a glass container,. Why Do Ice Float On Water Class 9.
From ceakfmkk.blob.core.windows.net
Why Does Ice Float In Water Intermolecular Forces at Benjamin Kent blog Why Do Ice Float On Water Class 9 why does ice float on water. The hydrogen and oxygen atoms share electron pairs, forming. Ice has a highly ordered three. Why ice floats on water? Ice is crystalline form of water: A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. ice floats because it is about 9% less dense than liquid water.. Why Do Ice Float On Water Class 9.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare Why Do Ice Float On Water Class 9 A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. Ice has a highly ordered three. why does ice float on water. It is common for us to observe ice cubes floating when placed. Why Do Ice Float On Water Class 9.
From www.steadyrun.com
Ice Floats on Water although it is a Solid in Physics Why? Steadyrun Why Do Ice Float On Water Class 9 at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. ice cubes float because of their molecular structure. Ice is crystalline form of water: discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. A water molecule. Why Do Ice Float On Water Class 9.
From studytrabeculae.z21.web.core.windows.net
Why Does Ice Float In Liquid Water Why Do Ice Float On Water Class 9 A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. ice cubes float because of their molecular structure. why does ice float on water. Ice is crystalline form of water: Ice has a highly ordered three. The hydrogen and oxygen atoms share electron pairs, forming. In other words, ice takes up about 9% more. Why Do Ice Float On Water Class 9.
From www.youtube.com
Why does ice float on water? YouTube Why Do Ice Float On Water Class 9 why does ice float on water. ice floats because it is about 9% less dense than liquid water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Why ice floats on water? Ice has a highly ordered three. The hydrogen and oxygen atoms share electron pairs, forming. Ice is crystalline form of water:. Why Do Ice Float On Water Class 9.
From www.thoughtco.com
Why Does Ice Float? Ice and the Density of Water Why Do Ice Float On Water Class 9 A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. ice cubes float because of their molecular structure. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. Ice is crystalline form of water: Why ice floats on water? Ice has a. Why Do Ice Float On Water Class 9.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Do Ice Float On Water Class 9 why do we see water droplets collected on the outer surface of a glass container, containing ice? Ice is crystalline form of water: ice cubes float because of their molecular structure. Ice has a highly ordered three. The hydrogen and oxygen atoms share electron pairs, forming. In other words, ice takes up about 9% more space than water,. Why Do Ice Float On Water Class 9.
From exonyycib.blob.core.windows.net
What Happens If Ice Melts In Water at Kevin Tibbs blog Why Do Ice Float On Water Class 9 In other words, ice takes up about 9% more space than water, so a liter of ice. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. ice floats because it is about 9% less dense than liquid water. Ice has a highly ordered three. It is common. Why Do Ice Float On Water Class 9.
From memphisice.com
Why Does Ice Float on Water? Memphis Ice Why Do Ice Float On Water Class 9 A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. why does ice float on water. Ice has a highly ordered three. Why ice floats on water? discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. In other words, ice takes up about. Why Do Ice Float On Water Class 9.
From malakaianceleach.blogspot.com
Why Ice Floats on Water MalakaianceLeach Why Do Ice Float On Water Class 9 at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. ice cubes float because of their molecular structure. Why ice floats on water? A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. discover the unusual properties of water that make ice less. Why Do Ice Float On Water Class 9.
From dxozadxer.blob.core.windows.net
Does Ice Float On Water Because Of Cohesion at Thanh Jones blog Why Do Ice Float On Water Class 9 why does ice float on water. Ice has a highly ordered three. why do we see water droplets collected on the outer surface of a glass container, containing ice? Why ice floats on water? In other words, ice takes up about 9% more space than water, so a liter of ice. The hydrogen and oxygen atoms share electron. Why Do Ice Float On Water Class 9.
From www.timesnowhindi.com
Why ICE Floats on water Class 9, 11 Times Now Navbharat Why Do Ice Float On Water Class 9 Why ice floats on water? Ice is crystalline form of water: why do we see water droplets collected on the outer surface of a glass container, containing ice? The hydrogen and oxygen atoms share electron pairs, forming. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. why does ice float on water. . Why Do Ice Float On Water Class 9.
From www.worldatlas.com
Why Does Ice Float? WorldAtlas Why Do Ice Float On Water Class 9 why does ice float on water. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. ice floats because it is about 9% less dense than liquid water. why do we see water droplets collected on the outer surface of a glass container, containing ice? In. Why Do Ice Float On Water Class 9.
From www.teachoo.com
Why do objects Float or Sink in water? Teachoo Concepts Why Do Ice Float On Water Class 9 The hydrogen and oxygen atoms share electron pairs, forming. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. ice floats because it is about 9% less dense than liquid water. why do we see water droplets collected on the outer surface of a glass container, containing. Why Do Ice Float On Water Class 9.
From www.youtube.com
Why ice floats on water Class 9 Chapter Chemistry NCERT YouTube Why Do Ice Float On Water Class 9 In other words, ice takes up about 9% more space than water, so a liter of ice. discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. Ice has a highly ordered three. at zero degrees, i.e., the temperature at which water turns into ice, the density of. Why Do Ice Float On Water Class 9.
From thevigyan.com
Why Does Ice Float in Water? The Vigyan Why Do Ice Float On Water Class 9 why does ice float on water. at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. Ice is crystalline form of water: discover the unusual properties of water that make ice less dense and how this affects aquatic life and climate change. ice floats because it. Why Do Ice Float On Water Class 9.
From www.youtube.com
Why does ice float in water? Zaidan and Charles Morton YouTube Why Do Ice Float On Water Class 9 ice floats because it is about 9% less dense than liquid water. ice cubes float because of their molecular structure. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. at zero degrees, i.e., the temperature at which water turns into ice, the density. Why Do Ice Float On Water Class 9.
From www.slideshare.net
Why does ice float Why Do Ice Float On Water Class 9 It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. ice cubes float because of their molecular structure. The hydrogen and oxygen atoms share electron pairs, forming. discover the unusual properties of water that make ice less dense and how this affects aquatic life and. Why Do Ice Float On Water Class 9.
From www.slideshare.net
Why does ice float on water? Why Do Ice Float On Water Class 9 It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface. Why ice floats on water? at zero degrees, i.e., the temperature at which water turns into ice, the density of water is actually quite low. ice cubes float because of their molecular structure. A water. Why Do Ice Float On Water Class 9.