What Is A Buffer In The Human Body . A solution used to stabilize the ph (acidity) of a liquid. The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. The higher the buffer concentration, the greater. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. What causes changes in blood ph?
from labpedia.net
The higher the buffer concentration, the greater. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. A solution used to stabilize the ph (acidity) of a liquid. What causes changes in blood ph? It takes only seconds for the chemical. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1.
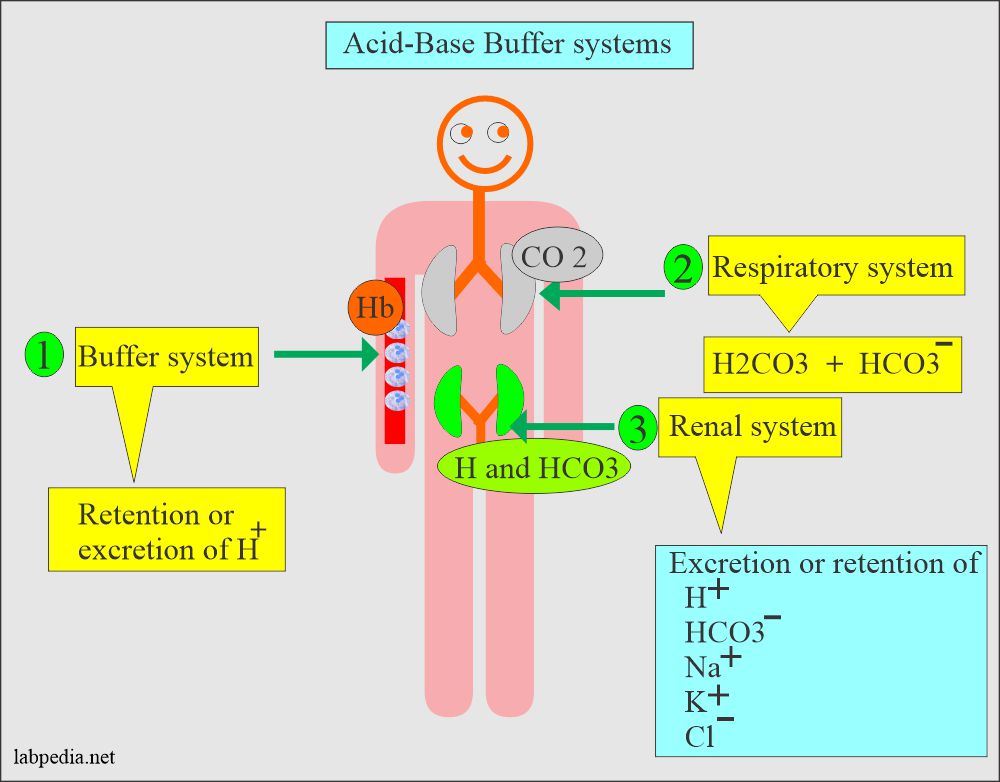
Acidbase Balance Part 2 Introduction of AcidBase Balance
What Is A Buffer In The Human Body The higher the buffer concentration, the greater. It takes only seconds for the chemical. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The higher the buffer concentration, the greater. A solution used to stabilize the ph (acidity) of a liquid. What causes changes in blood ph? Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and.
From www.youtube.com
What is Buffer ? Why Buffer and TriState Buffers are used in Digital What Is A Buffer In The Human Body A solution used to stabilize the ph (acidity) of a liquid. The buffer systems in the human body are extremely efficient, and different systems work at different rates. What causes changes in blood ph? A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1.. What Is A Buffer In The Human Body.
From www.slideserve.com
PPT Bronsted Lowry PowerPoint Presentation ID5171770 What Is A Buffer In The Human Body Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The. What Is A Buffer In The Human Body.
From www.slideshare.net
Buffers in the body What Is A Buffer In The Human Body The buffer systems in the human body are extremely efficient, and different systems work at different rates. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions,. What Is A Buffer In The Human Body.
From present5.com
Fundamentals of General Organic and Biological Chemistry 6 What Is A Buffer In The Human Body Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. What causes changes in blood ph? It takes only seconds for the chemical. A solution used to stabilize the ph (acidity) of a liquid. The higher the. What Is A Buffer In The Human Body.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 What Is A Buffer In The Human Body Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. What causes changes in blood ph? The buffer systems in the human body are extremely efficient, and different systems work at different rates. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. The higher the buffer. What Is A Buffer In The Human Body.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 What Is A Buffer In The Human Body Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. The higher the buffer concentration, the greater. It takes only seconds for the chemical. The buffer systems in the human body are extremely efficient, and different systems. What Is A Buffer In The Human Body.
From poorvi77.blogspot.com
Poorveez ParadyzMedimuseion major buffer systems in the body What Is A Buffer In The Human Body A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The higher the buffer concentration, the greater. Several substances serve as buffers in the body, including cell. What Is A Buffer In The Human Body.
From ppt-online.org
Disorders of metabolism. (Subject 9) презентация онлайн What Is A Buffer In The Human Body Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. The higher the buffer concentration, the greater. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. It takes only seconds for the chemical. Mical called. What Is A Buffer In The Human Body.
From labpedia.net
Acidbase Balance Part 2 Metabolic acidosis, and Metabolic What Is A Buffer In The Human Body The higher the buffer concentration, the greater. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. A solution used to stabilize the ph (acidity) of a liquid. A buffer is a solution containing substances. What Is A Buffer In The Human Body.
From labpedia.net
Acidbase Balance Part 2 Introduction of AcidBase Balance What Is A Buffer In The Human Body Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. What causes changes in blood ph? The buffer systems in the human body are extremely efficient, and different systems work at different rates. The higher the buffer concentration, the greater. A solution used to stabilize the ph (acidity) of a liquid. A buffer is a. What Is A Buffer In The Human Body.
From www.studocu.com
Buffers OF Human BODY Date BUFFERS IN HUMAN BODY BLOOD BUFFERS What Is A Buffer In The Human Body It takes only seconds for the chemical. A solution used to stabilize the ph (acidity) of a liquid. The higher the buffer concentration, the greater. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. Several substances serve as buffers in the body, including. What Is A Buffer In The Human Body.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Is A Buffer In The Human Body The higher the buffer concentration, the greater. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. It takes only seconds for the chemical. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The buffer. What Is A Buffer In The Human Body.
From www.youtube.com
Buffer action in the blood YouTube What Is A Buffer In The Human Body What causes changes in blood ph? The higher the buffer concentration, the greater. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. A solution used to stabilize the ph (acidity) of a liquid. Several substances serve as buffers in the body, including cell. What Is A Buffer In The Human Body.
From urbanoak.co
Manscaped Body Buffer And Body Wash Detailed Review 2023 What Is A Buffer In The Human Body A solution used to stabilize the ph (acidity) of a liquid. The higher the buffer concentration, the greater. What causes changes in blood ph? It takes only seconds for the chemical. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. Several substances serve. What Is A Buffer In The Human Body.
From www.slideshare.net
Buffer system What Is A Buffer In The Human Body A solution used to stabilize the ph (acidity) of a liquid. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The higher the buffer concentration, the greater. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate. What Is A Buffer In The Human Body.
From www.pinterest.com
Image result for bicarbonate buffer system Medical student motivation What Is A Buffer In The Human Body A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. It takes only seconds for the chemical. The buffer systems in the human body are extremely efficient, and different. What Is A Buffer In The Human Body.
From medizzy.com
Buffer System in Body MEDizzy What Is A Buffer In The Human Body What causes changes in blood ph? Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. A solution used to stabilize the ph (acidity) of a liquid. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. The higher the buffer concentration, the greater. A buffer is. What Is A Buffer In The Human Body.
From study.com
Quiz & Worksheet Buffer Systems in Humans What Is A Buffer In The Human Body A solution used to stabilize the ph (acidity) of a liquid. It takes only seconds for the chemical. The higher the buffer concentration, the greater. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. What causes changes in blood ph? A buffer is a solution containing substances which have the ability. What Is A Buffer In The Human Body.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2821926 What Is A Buffer In The Human Body Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. A solution used to stabilize the ph (acidity) of a liquid. It takes only seconds for. What Is A Buffer In The Human Body.
From exoxpkges.blob.core.windows.net
Buffer Solutions Explained at Micaela Gregory blog What Is A Buffer In The Human Body It takes only seconds for the chemical. A solution used to stabilize the ph (acidity) of a liquid. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. A buffer is a solution containing substances which have the. What Is A Buffer In The Human Body.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance What Is A Buffer In The Human Body Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. The higher the buffer concentration, the greater. The buffer systems in the human body are extremely efficient, and different systems work at different rates. A buffer is a solution containing substances which have the ability to minimise changes in ph when an. What Is A Buffer In The Human Body.
From www.pinterest.com.au
Pin on Paramedici What Is A Buffer In The Human Body The higher the buffer concentration, the greater. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. A solution used to stabilize the ph (acidity) of a liquid. The buffer systems in the human body are extremely efficient, and different systems work at different rates. A buffer is a solution containing substances which have the. What Is A Buffer In The Human Body.
From www.aakash.ac.in
Acidic Buffer Solution Definition, Calculation & Applications What Is A Buffer In The Human Body What causes changes in blood ph? A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The higher the buffer concentration, the greater. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. It takes only seconds for the. What Is A Buffer In The Human Body.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and What Is A Buffer In The Human Body The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. Mical called adenosine triphosphate (atp) that consists of an adenine. What Is A Buffer In The Human Body.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching What Is A Buffer In The Human Body It takes only seconds for the chemical. What causes changes in blood ph? The buffer systems in the human body are extremely efficient, and different systems work at different rates. A solution used to stabilize the ph (acidity) of a liquid. The higher the buffer concentration, the greater. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded. What Is A Buffer In The Human Body.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts What Is A Buffer In The Human Body Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. The buffer systems in the human body are extremely efficient, and different systems work at different rates. What causes changes in blood ph? A solution used to stabilize the ph (acidity) of a liquid. It takes only seconds for the chemical. The higher the buffer. What Is A Buffer In The Human Body.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation What Is A Buffer In The Human Body A solution used to stabilize the ph (acidity) of a liquid. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The higher the buffer concentration, the greater. What causes changes in blood ph? Several substances serve as buffers in the body, including cell. What Is A Buffer In The Human Body.
From www.slideshare.net
Buffers in the body What Is A Buffer In The Human Body It takes only seconds for the chemical. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The higher the buffer concentration, the greater. What causes. What Is A Buffer In The Human Body.
From golifescience.com
What are the Physiological buffers and Systems in humans? What Is A Buffer In The Human Body What causes changes in blood ph? It takes only seconds for the chemical. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. The higher the buffer concentration, the greater. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and. A buffer is a solution containing substances. What Is A Buffer In The Human Body.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every What Is A Buffer In The Human Body The higher the buffer concentration, the greater. A solution used to stabilize the ph (acidity) of a liquid. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate. What Is A Buffer In The Human Body.
From dxorrxiwe.blob.core.windows.net
Buffer In Body Fluids at Judith Grunwald blog What Is A Buffer In The Human Body What causes changes in blood ph? The higher the buffer concentration, the greater. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Several substances serve as. What Is A Buffer In The Human Body.
From www.expii.com
Ion Balance — Regulation & Homeostasis Expii What Is A Buffer In The Human Body The higher the buffer concentration, the greater. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. It takes only seconds for the chemical. Several substances serve as buffers. What Is A Buffer In The Human Body.
From www.slideserve.com
PPT 1. Body Fluid Compartments PowerPoint Presentation, free download What Is A Buffer In The Human Body A solution used to stabilize the ph (acidity) of a liquid. The higher the buffer concentration, the greater. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Mical called adenosine triphosphate (atp) that consists of an adenine nucleotide bonded to three phosphates. A buffer is a solution containing substances which have the. What Is A Buffer In The Human Body.
From kyvokedymolyb.modellervefiyatlar.com
Acid Base Buffers What Is A Buffer In The Human Body The buffer systems in the human body are extremely efficient, and different systems work at different rates. The higher the buffer concentration, the greater. It takes only seconds for the chemical. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. A solution used. What Is A Buffer In The Human Body.
From fblt.cz
7. AcidBase Balance • Functions of Cells and Human Body What Is A Buffer In The Human Body It takes only seconds for the chemical. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The higher the buffer concentration, the greater. A solution used to stabilize the ph (acidity) of a liquid. What causes changes in blood ph? Several substances serve. What Is A Buffer In The Human Body.